CalQlata's Technical Definitions
This page is provided as a list of definitions for all the technical terms used throughout this website. However, as it is also a useful technical dictionary, it is important that it is error-free. Therefore CalQlata would appreciate its users pointing out any such errors along with the corrected text and/or images. Thank you!
High definition values for all constants referred to in this definition page are provided in CalQlata's UniQon calculator.
|
1-D (line) |
A one dimensional shape: a straight line |
|
2-D (plane) |
A two dimensional shape: a planar or 'flat' shape that becomes invisible if viewed 'edge-on' |
|
3-D (body) |
A three dimensional shape: a shape with volume |
|
Acceleration (a) |
Rate of change of velocity. This value may be interpreted as the second derivative of the distance between two points in a journey that may described mathematically thus: where: see also Linear Acceleration, Angular Acceleration and Rotational Acceleration |
|
Achiral |
Opposite images or systems that are mirrored versions of each other. Achiral systems (or images) are symmetrical. |
|
Acid |
A corrosive substance at the low end of the pᴴ scale (0<7) Acids turn blue litmus paper pink but do not affect the colour of pink litmus paper. The acidic component of water is H⁺. |
|
Acute (angle) |
An angle between 0° and 90° |
|
Added Mass Coefficient |
A coefficient that represents the additional mass of liquid or gas displaced by a body in which it is immersed, and/or relative to which which it moves: Whilst there are certain circumstances where an added mass coefficient can exceed 1.0, it is a rare occurrence that is invariably due to complex shapes or close proximity to structures or terrain. see Added Drag-technical help |
|
Adiabatic |
A process that generates a variation in the volume, pressure and/or temperature of a substance, but no heat is added to the system (from outside) or lost to its surroundings (from inside) see Thermodynamics |
|
Aerobe |
An organism that can only live in the presence of free oxygen |
|
Aerobic |
The breathing (respiration) of free oxygen and its conversion to energy |
|
Aerofoil |
see airfoil |
|
Age Hardening |
A hardening process that occurs with time with no added chemicals or energy see also Marageing |
|
Air (the Earth) |
The earth's atmosphere is divided into the following layers: 99.999622% of the Earth's air (atmosphere) comprises the following gases: Properties# @ sea level and 273.15K: |
|
Airfoil |

A device used to induce unequal pressure on both faces for the purpose of inducing lateral movement or force. |
|
Alkaline |
A base substance at the high end of the pᴴ scale (7<14) Alkaline turns pink litmus paper blue but does not affect the colour of blue litmus paper The alkaline component of water is OH⁻. |
|
Alloy |
An alloy is a compound (mixture) of more than one metal. A steel alloy normally refers to an iron compound with more than trace amounts of elements other than and in addition to: carbon, manganese, phosphorus and sulphur. see alloy steel and copper alloys |
|
Alpha Iron |
Pure iron (< 1,183 K) with a body centre cubic crystal structure will contain up to 0.001% carbon in solution see carbon steel |
|
Alpha Particle |
A kinetic proton ejected from an atom's nucleus as a result of fissionable decay. Because neutrons are created in pairs (two electrons per shell) they are also split in pairs; two protons and two electrons. Note: Fissionable decay also releases neutron energy in form of heat if the proton is not released from its nucleus after splitting. This can occur to either one or both of the protons released simultaneously. |
|
Alternating Current (AC) |

The flow of electrical energy in the EME radiated by the 'shell-1' proton-electron Pairs between atoms in an electrical conductor. AC current is normally supplied by a generator and used for high-power applications (>100W) such as free-standing industrial and domestic equipment. see also DC (electrical), Apparent Power, True Power, Phase Angle and Power Factor |
|
Alternator |
An alternator uses magnets and coils of copper wire to convert rotary motion into AC electricity. |
|
Altitude |
Height above a designated surface. It is most frequently used to define the height above sea-level at the earth's surface. |
|
Ampere (A) |
Unit of electrical current. One Ampere is equal to one coulomb (of electrons) passing through a conductor in one second. |
|
Amorphous |
Random arrangement of particles or components. |
|
Amplitude |

A displacement that represents half the total fluctuation in a complete cycle, or the maximum deflection of a beam or particle from a central datum |
|
Anaerobic |
Respiration that does not use free oxygen to generate energy |
|
Angle of Repose (θ) |
The smallest angle at which an object will slide down a flat surface under its own weight (or contact force) excluding the effects of stiction, chemistry or magnetism |
|
Angular Acceleration |

The rate of change of angular velocity; ω/s = θ/s². This value may be interpreted as the second derivative of the angular rotation of a point on the circumference of a circle, which may described mathematically thus: where: see also Linear Acceleration and Rotational Acceleration |
|
Angular Velocity |

The rate of change of angular rotation; θ/s. This value may be interpreted as the first derivative of the angular rotation of a point on the circumference of a circle, which may described mathematically thus: where: see also Tangential Velocity, Linear Velocity and Rotational Velocity |
|
Anion |
A negatively charged ion. An atom or molecule that has gained excess electron(s) and is therefore attracted to an anode in an electrolyte |
|
Anisotropic |
A description of material properties that vary with direction. |
|
Anneal |
To anneal a carbon steel is to soften it. Forged carbon steel is annealed by heating it to between 500°C and 700°C and holding it at this temperature to allow recrystallisation to occur, followed by artificial cooling. Cast carbon steel is annealed by heating it above 723°C and holding it at this temperature to allow all the material to transform to austenite, followed by artificial cooling. Artificial cooling means more slowly than it would cool in still air and is normally carried out in an oven. see carbon steel |
|
Anode |
A positively charged electrode that loses cations to a negatively charged electrolyte (corrodes) |
|
Anti-Knocking Agent |
The anti-knocking agent used in petrol in most developed countries today is AK-33-X (cyclopentadienyl-Manganese-Tricarbonyl) The anti-knocking agent used for petrol in the past was TEL (Tetra-Ethyl-Lead:64%, Ethylene Bromide:25%, Ethylene Chloride:9% and Methylene Dye:2%) |
|
Aphelion |

The furthest point an orbiting body gets from its force-centre Whilst this term can also be used instead of Apogee (i.e. it means the same thing), it is normally used to describe the point at which the distance between sun and its orbiting planet is greatest a = half the width of the ellipse see also Perigee and Perihelion |
|
Apogee |

The furthest point an orbiting body gets from its force-centre Whilst this term can also be used instead of Aphelion (i.e. it means the same thing), it is normally used to describe the point at which the distance between the earth and its orbiting moon is greatest a = half the width of the ellipse see also Perigee and Perihelion |
|
Apparent Power (electrical) |
Apparent power is the maximum theoretical power of an alternating current (peak current multiplied by peak voltage) and is normally expressed in terms of 'VA' or 'kVA'. see also AC, True Power, Phase Angle and Power Factor |
|
Arc |
A curved line that forms part of the circumference of a circle |
|
Area Moment |
A property of the cross-sectional area of any structural body that defines its ability to resist bending or torsion see also Second Moment of Area, Moment of Inertia and Polar Moment of Inertia |
|
Armature |
A low-reluctance ferro-magnetic body (keeper) for temporarily bridging the poles of a permanent magnet to reduce the leakage field and preserve magnetisation in DC motors and generators. |
|
Aspect Ratio |
The ratio between two physical dimensions Aspect ratio's may also be reversed according to user preference. I.e. width to length (W:L) |
|
Astroid (curve) |

A special case of hypocycloid curve generated by a point on the circumference of a circle rolled around the the inside of a stationary circle four times the diameter without slipping. see also Hypocycloid |
|
Asymmetry |
Opposite images that are not symmetrical. Asymmetrical systems are chiral systems. |
|
Atmospheric Pressure |
Atmospheric pressure refers to the pressure surrounding your subject or location. The units used for defining pressure above atmospheric are normally given the suffix 'a' or 'atm' Atmospheric pressure will vary according to the density of the surrounding gases (air). At sea-level and on dry land, atmospheric pressure is approximately equal to 14.7psi or 0.10135294N/mm² When specifying absolute pressures (those that include atmospheric) you should qualify the units with 'a' or 'atm'; |
|
Atom |
The generic term for a collection of proton-electron pairs the protons of which are inside the innermost electron orbital shell. The number of proton-electron pairs is allocated an atomic number. Natural atoms range between atomic numbers of 1 to 92. see The True Atom |
|
Atomic Element |
A collection of deterium and tritium atoms, the ratio of which gives an atomic element its neutronic ratio. Element is the generic term for an atom that includes its neutrons. |
|
Atomic Mass Unit |
One 12ᵗʰ of the mass of a pure carbon atom (¹²C) One amu is the reciprocal of Avogadro's number |
|
Atomic Number |
|
|
Atomic Particle |
The proton and the electron. A neutron is not an atomic particle. |
|
Austenite |
Face centre cubic iron, which occurs at temperatures higher than 723°C (also called Gamma Iron). At 723°C austenite can absorb up to 0.83% carbon within its crystal structure and hold another 0.87% in solution. see Carbon Steel |
|
Autoclave |
A thick-walled pressure vessel used for heating gases under pressure Autoclaves are generally used for facilitating chemical reactions that require carefully controlled heat and pressure over a period of time, such as using steam to vulcanise rubber. |
|
Avogadro's Number |
The number of atoms in 12 grams of pure carbon (¹²C): Historically, this value has been specified as; NA = 6.02214129E+23/mol Avogadro's number is also the reciprocal of one atomic mass unit in grammes see also mole |
|
Axial |
Along the longitudinal axis. |
|
Azimuthal Quantum Number (ℓ) |
The second in a set of quantum numbers that describes the angular momentum (or shape) of an atomic orbital The azimuthal quantum number of an electron can only be a positive integer: i.e. 1, 2, 3, 4, 5, 6, 7, etc. The orbital shape options in any shell are normally identified with the letters; s, p, d, f, etc. Whereas the azimuthal quantum numbers for each shape are defined thus: s(ℓ=1), p(ℓ=2), d(ℓ=3), f(ℓ=4), etc. At least one other quantum number must be different for each electron in the same shell with the same azimuthal quantum number |
|
Bar |
Alternative term for a beam but usually used for those with a circular (or regular) cross-section. |
|
Baryon |
A family of composite particles made of three quarks which includes protons and neutrons Baryons are strongly interacting fermions and part of the hadron particle family |
|
Baryon Number |
One third of the number of quarks the particle contains For example: |
|
Basic Oxygen Process |
A steel making procedure It is essentially the same as the Bessemer Converter but blows pure oxygen instead of air through the nozzles. It produces better quality steel and has largely replaced all Bessemer 'air-blowing' systems. |
|
Basic Temperature |
The lowest possible Temperature; Ṯₓ = 2.04274907568265 K |
|
Bead Wire |
A wire approximately 1mm diameter manufactured from 'Plow Steel', which is then drawn down to form the filaments used in wire ropes The term 'Bead Wire' comes from its use in untreated form to support the rim (or bead) of a tyre |
|
Beam |
An elongated body to which a transverse load is applied |
|
Bearing Strength |
The ability of a body of viscous matter to support pressure without collapse or plastic deformation 'Bearing Stress' is the measurement designation for this property |
|
Bending Moment |
A force applied at a distance that induces bending in a body |
|
Berm |
A heap or pile of material (soil, sand, rocks, etc.) normally positioned to protect and/or prevent movement of a structural object (e.g. pipeline) |
|
Bessemer Converter |
A steel making furnace A large steel vessel supported on pivots with one spouted opening in the top. Air is blown through a number of nozzles in the flat base to increase applied heat. Oxygen in the air is converted to carbon-dioxide This process is gradually being replaced by the Open Hearth furnace |
|
Beta Particle |
An electron ejected from an atom's nucleus as a result of fissionable decay. Because neutrons are created in pairs (two electrons per shell) they are also split in pairs; two protons and two electrons. Note: In reality, fissionable decay also results in the release of neutron energy in form of heat if the electron's proton partner is not released from its nucleus after splitting. This can occur to either one or both of the electrons released simultaneously. |
|
Birdcaging |
The term used to describe the unwinding and opening up of a wrapped assembly such as a wire rope or HPHT flexible pipe when it is axially compressed or twisted against the helical lay direction |
|
Bisector |
A line that passes through the centre of another line or object thereby dividing it exactly in two A perpendicular Bisector is a line that passes through the centre of another line or object at right-angles to it |
|
Black-Hole |
A fictitious entity with sufficient mass to generate the gravitational energy that will prevent photons from escaping its surface see Black Holes and Dark Matter |
|
Body Centre Cubic (bcc) |

Describes the lattice arrangement of atoms within a metal. The smallest crystal of this metal will contain nine closely packed atoms, eight of which are located at each corner of a cube and one in its centre. |
|
Bohr Radius |
See Rydberg radius |
|
Boiling Point |
The temperature at which a liquid vapourises. |
|
Boltzmann Constant |
Defines the relationship between energy and temperature in an ideal gas, it is measured in Joules per Kelvin or ft.lbf per Rankine, and is calculated thus: However; a modified version of KB gives us the relationship between energy and temperature in a proton-electron pair; Boltzmann Constant is also called the Stefan-Boltzmann constant as the concept was originally derived by Josef Stefan and later improved by Ludwig Boltzmann |
|
Boson |
Any particle that obeys Bose-Einstein statistics 5 are known; Higgs, photon, gluon, W & Z Photon, gluon, W & Z are Gauge Bosons Bosons are normally associated with force and have integer spin properties: 1, 2, 3, 4, 5, etc. Several bosons with the same energy can occupy the same quantum state, i.e. several bosons can occupy the same place in space. |
|
Bottom Quark |
Also known as the beauty quark, it has more than four times the mass of a proton and is part of the third generation of matter The bottom quark is classified as a fermion mᵣ=7.451526101E−24g [4.18GeV/c²], lifetime≈1E-12s, Q=-⅓e, Iz=-½ |
|
Brass |
The term used to describe a copper-zinc alloy The most common ratios (Cu%:Zn%) are: 95:5, 90:10, 85:15, 80:20, 75:25, 70:30, 65:35 & 60:40 As zinc content rises (see note below): 60:40 Brass is known as Munz Metal Note: this relationship holds true up to about 35% zinc after which the alloy changes phase from alpha brass to alpha-beta brass and strength drops slightly |
|
British Thermal Unit |
The heat energy required to raise the temperature of one-pound (avoirdupois) of water by one degree Farenheit. Due to the erroneous belief that specific heat capacity varies with temperature, the historic values for this constant are; The genuine (actual) British thermal unit: see also Calorie |
|
Brittle |
Any matter with unusually high viscosity The manifestation of brittleness is a body's susceptibility to shatter before achieving plastic deformation. |
|
Bronze |
The term normally used to describe copper-tin alloy. The most common ratios of copper-tin alloys (Cu%:Sn%) are: 98.75:1.25, 95:5, 92:8 & 90:10 As tin content rises: |
|
Brown & Sharpe Taper |
A conical shaft and mating sleeve for machine-part self-holding applications that optimises assembly load, holding capacity and easy release (without damage). The tapered shaft is normally driven by an End-Tang (or Key) or a Longitudinal Key. Different size numbers (0 to 16) relate to different engaged lengths (1-5/16" to 9") As each size is based upon standard fraction lengths and diameters, not all tapers will have exactly the same taper (angle ≈ 2.4°). The tapers range between 0.04167 and 0.043 inches per inch (2.39° to 2.46°) The Brown & Sharpe Taper is used for similar applications to the Morse and Jarno Tapers |
|
Bulk Modulus |
Relationship between a 2-D stress (load per unit area) and the resultant 3-D strain in a body. the general formula for this property is; The bulk modulus for compressible substances such as gases is sometimes specified as follows; |
|
Buoyancy |
The vertical (anti-gravity) force exerted by a body immersed in a fluid A body with a lower density than its surrounding fluid will float or rise (positive force) A body with the same density than its surrounding fluid will settle mid-column (zero force) A body with a greater density than its surrounding fluid will sink (negative force) |
|
Bush (m/c) |
A hollow cylindrical sleeve or spacer between a shaft and its housing. |
|
Calorie |
The heat energy required to raise the temperature of one-gramme of water by one degree Celsius. Due to the erroneous belief that specific heat capacity varies with temperature, the historic values for this constant are; The genuine (actual) calorie: see also British Thermal Unit |
|
Capacitance |

Capacitance (the unit of measurement is the 'Farad') is the resistance generated by the storage of Volts as current grows in an AC power supply. This voltage is slowly released as current falls. Current leads voltage C = Q/V |
|
Capstan |
A cylindrical drum about which a cord is wrapped to increase frictional resistance - between the cord and the drum - in order to maintain tension. The purpose of a capstan is to provide a high output tension for little or even no input tension, dependent upon the number of turns and the friction coefficient. A mechanical (or operational) capstan has an ratchet that allows the user to increase output tension by rotating the drum. |
|
Carbide |
A binary compound of carbon and a metal |
|
Carbon Dating |
The aging of matter by measuring the half-life of the carbon-14 atom. see Carbon Dating |
|
Carburise |
The process of transferring carbon into the surface of a carbon steel to case harden it. It is carried out by immersing or embedding the steel in a carbon rich substance and heating it to a temperature where the transfer of carbon atoms occurs. The longer this process is maintained, the greater the depth of hardening. |
|
Cardioid (curve) |

A special case of epicycloid curve generated by a point on the circumference of a circle rolled around the the outside of a stationary circle exactly the same size without slipping. |
|
Cartesian |

The definition of a vector using its linear dimensional relationship to the origin of a set of 2-D (x,y) or 3-D (x,y,z) axes see also Polar Co-Ordinates and Vector |
|
Case Harden |
A process during which only the outer skin (or case) of a carbon steel is hardened. The inner body of the material retains its original ductility/toughness. |
|
Cathode |
A negatively charged electrode that gains cations via a negatively charged electrolyte (gains matter) |
|
Cation |
A positively charged ion. An atom or molecule with a shortage of electron(s), which is therefore attracted to a cathode in an electrolyte |
|
Cementite |
A very hard and brittle iron carbide that forms when excess carbon cannot be held in solution in iron Pure cementite has the following physical properties: see Carbon Steel |
|
Central Force Motion |
The movement of an Orbital force-centre (e.g. star or planet) towards its satellites due to very high Potential energy in particularly massive satellites; e.g. Pluto and its moons. |
|
Centre of Buoyancy |
The centre of mass of the fluid displaced by an immersed body |
|
Centre of Gravity |
The only point on a line, shape or object that can be supported on the end of a pin without the line, shape or object tilting (also known as the centre of area or centre of mass) |
|
Centrifugal |
The outward action of a point or body in a circular motion about a centre-point or anchor. see also Centripetal |
|
Centrifugal Acceleration |
The positive radial (outward) acceleration of an orbiting body directed away from its centre of rotation (or force centre) (the opposite of centripetal acceleration) see Laws of Motions |
|
Centrifugal Force |
Centrifugal acceleration of a body multiplied by its mass (the opposite of centripetal force) see also Central Force Motion and Force |
|
Centripetal |
The inward reaction of a point or body in a circular motion about a centre-point or anchor. see also Centrifugal |
|
Centripetal Acceleration |
The negative radial (inward) acceleration of an orbiting body directed towards its centre of rotation (or force centre) (the opposite of centrifugal acceleration) |
|
Centripetal Force |
Centripetal acceleration of a body multiplied by its mass (the opposite of centrifugal force) see also Gravitational Force, Central Force Motion and Force |
|
CFC |
Chlorofluorocarbon - an organic gaseous compound used as a refrigerant and as a pressuriser in aerosol cans. CFCs have mistakenly been blamed as the cause of a hole in an ozone layer |
|
Charge Capacity |
The quantity of electrical energy that can be absorbed by a given mass of substance per unit temperature. Charge capacity = electrical charge {C} x specific charge capacity {J / K.C}. Charge Capacity of a proton-electron pair: The units of measurement are J/K, Btu/°R, W.s/K, N.m/K, lbf.ft/°R, etc. see Charges |
|
Charge Density |
see radiation |
|
Charm Quark |
The third most massive of all the quarks and part of the second generation of matter The charm quark is classified as a fermion mᵣ=2.299633653E−24g [1.29GeV/c²], Q=⅔e, Iz=½ |
|
Chiral |
Opposite images or systems that are not mirrored versions of each other. Chiral systems (or images) are asymmetrical. |
|
Chord |
A straight line that joins both ends of an Arc |
|
Circle |

The locus generated by rotating a point at a constant distance (R) around a central origin (O) Where; R² = x² + y² see Elliptical Curves |
|
Circulation |
The angular momentum of the fluid in a vortex. |
|
Clamped End |
A fixed support |
|
Clutch |
A frictional connection between a drive-shaft and a driven-shaft. The principal advantages of a clutch are, the ability to: |
|
Coefficient |
 A ratio of similar properties, and therefore dimensionless. Using drag coefficient as an example; Coefficients are established through mathematics or experimentation. see also Factor |
|
Coefficient of Expansion |
A factor representing the linear relationship between temperature and dimensional change in metals There are coefficients for; where see Thermodynamics |
|
Coefficient of Friction |
A factor representing the linear relationship between the contact force of two surfaces and their relative sliding resistance μ = the tangent of the Angle of Repose |
|
Column |
An elongated body onto which an axial load is applied |
|
Combined Stress |
Normally interchangeable with equivalent stress When differentiating between two different types of combined stress, CalQlata uses the following specific definitions: see Combined Stress |
|
Commutator |
 A means whereby induced current may be transmitted between a rotating armature and a stationary electrical circuit. Comprises a split slip-ring or bush that is electrically connected to a conductor within, and attached to, a rotating armature. The split in the ring (or bush) is to ensure that the electro-magnetic current travels in the same direction through the conductor (as the armature rotates). |
|
Compressibility Factor |
Defines a gas's ability to deform under compression It is calculated as follows: See Pipe Flow+ (Fig 1) |
|
Concave |

The inside surface of a curved plane. |
|
Conductance |
The reciprocal of Resistance G = 1/Ω = I/V {C² / J.s} |
|
Conduction |
see heat |
|
Conductivity |
The reciprocal of Resistivity σ = 1/ρ = 1 / Ω.m {C² / J.s.m} |
|
Conductor |
A material that allows the transfer of its outermost [shell] electrons between adjacent atoms when exposed to a potential difference. This term is relative. You can have a good conductor and a bad conductor. The better the conductor, the easier it is to release its outermost [shell] electrons from their proton-partners. see also Resistor |
|
Conic |

A curve generated by a plane cutting through a circular cone The curve is defined by the locus of a point, whose distance from a fixed point (called the focus) is a constant ratio to its distance from a fixed line (called the directrix). This ratio is called the eccentricity (e) If e = 0 the curve is circular The upper cone is a nappe and the lower cone is another nappe |
|
Constant of Motion |
Newton's orbital momentum without the mass component, which applies equally to satellites (celestial - elliptical and atomic - circular), and their force-centres. Celestial (variable): Neutronic (constant): see Laws of Motion |
|
Constant of Proportionality |
Newton's dimensional orbital constant, which applies to every orbit encircling the same force-centre, but differs for each (force-centre). K = (2π)² / G.m = t²/a³ {s²/m³} The constants of proportionality for ... see Laws of Motion |
|
Convection |
see heat |
|
Convex |

The outside surface of a curved plane. |
|
Co-ordinate(s) |
Linear distance(s) between two points in space relative to a 3-dimensional axis system. |
|
Coriolis |
Effect: the directional influence on a vortex at the surface of a planet (e.g. the earth), which is defined by the relative rotational direction of the earth's surface spin and its moon’s orbit. This is why free (natural) vortices rotate in an anti-clockwise direction (positive rotation) in the earth’s northern hemisphere and clockwise (negative rotation) in the southern hemisphere. Force: the magnetic (gravitational) influence of the moon on the fluid vortex. This tends to be considerably less than the physical forces induced by fluid head, pressure, velocity, etc. but sufficient to influence [initialise] rotational direction. This force varies over the earth's surface according to latitude. The greatest forces occur at the earth's poles and the least (≈0) at the equator, where the switch between positive and negative rotation occurs. |
|
Corrosion |
Corrosion is the process whereby a metal is degraded and/or its atoms are removed chemically 'Rust' is a special case where oxidisation is the chemical process that occurs in iron via an electrolyte |
|
Coulomb |
The unit of electrical charge (or work): 1 C = 1 Amp.sec One Coulomb is approximately equal to the charge of 6.24150964505573E+18 electrons The pipeline analogy for Coulomb is the volume of fluid flowing along a pipe see also Voltage |
|
Coulomb's Constant |
Charles-Augustin de Coulomb's constant of proportionality for electrostatic force Coulomb's constant for a proton: k' = k/ξₘ See Coulomb's 'k' & magnetism |
|
Coulomb's Force |
The attractive or repulsive force between electrical charges. F = k.(e/R)² see Coulomb's 'k' & magnetism |
|
Coulomb's Law |
The force of attraction or repulsion (F) between two charged points is proportional to the product of their charges (Q₁.Q₂) and inversely proportional to the square of their separation distance multiplied by the relative permittivity (d².ε) of the medium: Because electricity is shared, Q₁ & Q₂ must be of equal magnitude; in other words; Coulomb's constant of proportionality (k) changes the above relationship to: Note: the relative permittivity at atomic level is generally regarded as 1.0 |
|
Coupling Ratio |
A universal constant that defines the ratio between magnetic charge (gravitational) force (Fₘ) and electrical charge force (Fₑ) φ = Fg/Fₑ = 4.40742111792333E-40 See Rydberg Atom |
|
Covalent Bond |
Historic explanation: 
which can only occur between electrons in the valence shell (including sub-shells) of atoms I.e. there must be an electron and a gap in the valence shell of both atoms for bonding to occur. For Example: Whilst covalent bonds normally occur between non-metal atoms (carbon, hydrogen, oxygen, nitrogen, etc.), they also occur between some metals and non-metals, e.g. beryllium and aluminium both form covalent bonds with chloride atoms. However, there are no valencies in atomic electron shell's, so the above explanation is hypothetical. |
|
Creep |
The gradual and continual movement or distortion of an object or substance under load over time. Typical substances susceptible to creep are those will little elasticity such as soils and polymers |
|
Critical Pressure (cp) |
The pressure at which a gas will change state to a liquid at its critical temperature. E.g.; However, given recent discoveries concerning the atom and the states of matter (Fig 2), 'critical states' can only be theoretical. |
|
Critical Temperature (ct) |
Gas: The temperature above which a gas cannot be liquefied by pressure alone. E.g; Magnets: The temperature (Curie temperature) above which magnets lose their magnetic properties. E.g; Solids: The temperature above which a solid changes state or undergoes a fundamental change of its properties However, given recent discoveries concerning the atom and the states of matter (Fig 2), 'critical states' can only be theoretical. |
|
Crooke's Tube |
A sealed, evacuated glass tube in which an electrode is installed at each end. A voltage is passed across the two electrodes that materialises in the form of a path of light. see The Error |
|
Cross-Section |
A two-dimensional plane through a three-dimensional body. |
|
Crucible Furnace |
A Crucible furnace is used to manufacture consistently high-quality steel. It is a very high-temperature melting process that isolates the steel from the fire in order to ensure total control over the carbon content. Modern steelmaking methods can achieve almost the same level of quality but much less expensively. |
|
Crystal (metals) |
A crystal of any metal is a single body (of any size) containing purely its own atoms (i.e. no impurities) all of which are arranged in prisms (trihedron, quadrahedron, pentahedron, hexahedron, heptahedron, etc.) with the same orientation and spacing in a consistent lattice structure. As a crystal grows, each face of every prism (or cube) in the lattice structure will also form the face of a neighbouring prism (or cube). see also Cubic, Body Centre Cubic, Face Centre Cubic, Hexagonal Close Packed, Rhombic and Tetra-Hedra |
|
Cubic |

Describes the lattice arrangement of atoms within a metal. The smallest crystal of this metal will contain eight closely packed atoms, each of which are located at each corner of a cube. |
|
Cubic Expansivity |
|
|
Curie Point |
The point at which a material changes to or from being ferromagnetic to paramagnetic or diamagnetic Some examples are provided below: These changes occur at temperatures at which an element alters its lattice structure. |
|
Current |
The the flow-rate of electrons along an electrical conductor due to a potential difference. The unit of measurement is the Ampere. The pipeline analogy for current is the rate of volumetric flow of the fluid along a pipe I = V.R |
|
Curvilinear Velocity |
|
|
Cycle |
A 'Process' that returns a heat engine to its original state |
|
Cycloid (curve) |

A cycloid is a curve generated by a point on the circumference of a circle that is rolled along a flat, straight surface without slipping. see also Trochoid, Epicycloid and Hypocycloid |
|
Dalton's Law |
The distribution, and therefore the pressure, of each same element gas in a mixture of gases is equal to the pressure it would exert if it occupied the same volume alone at the same temperature. This only works because ... see Dalton's Law |
|
Damage Ratio |
Also known as 'utilisation' (the reciprocal of safety factor) is a number that describes the likelihood that a mechanical component or material will survive its design life. A value of 'one' predicts that the component will fail on the last cycle of its design life with no safety factor. A number less than one reveals a component that will last longer than its design life whilst a number greater than one shows a component that will fail before the end of its design life |
|
Damping (vibration) |
The reduction or avoidance of harmonic excitation (resonance). |
|
Damping Factor |
The ratio of damping (C) to critical damping (Cc): ζ = C ÷ Cc ζ < 1.0 is considered under-damped |
|
de Broglie Wavelength |
The wavelength of a particle due to its momentum λ = h/p Planck's constant is actually incorrect, so the above calculation cannot work. |
|
Deflagration |
Sudden burst into flames see also Explosion |
|
Deformation Energy |
Energy absorbed/dissipated in the combined deformation of an impacting mass and the impacted system or body Elastic deformation energy: Uᴰ = ½ky² = ½Fy Plastic deformation energy: Uᴰ = Fy {m.a.y} Where: |
|
Degrees of Freedom |
There are six degrees of freedom; three vectors (or directions) according to the right-hand rule (x,y,z) and three rotations (xy,xz,yz) On a ship these six degrees of freedom are known as Surge, Sway, Heave and Yaw, Pitch, Roll respectively |
|
Delta Iron |
Pure iron (> 1,663 K) with a body centre cubic crystal structure |
|
Denier (d) |
The mass of fabric thread in grams per 9000 metres (length). Note: 9000 metres of silk thread has a mass of ≈1 gram |
|
Density (ρ) |
The mass per unit volume of a body or substance, measured in kg/m³, lb/in³ or g/cm³. e.g. 1.0 m³ of a substance with a density of 3000 kg/m³ will have a mass of 3000 kg. The ultimate (limiting) density is that of an atomic particle: see also Specific Gravity, Specific Volume and Newton's Mass |
|
Design Life |
The predicted period of time until immediately before a material or mechanical component is no longer expected to comply with its original design specification |
|
Deuterium (atom) |
A proton-electron pair with one neutron attached. |
|
Dew-Point |
The combined temperature and pressure at which the liquid in a vapour begins to condense An increase in pressure and/or a reduction in temperature of the vapour will cause this to occur |
|
Dezincification |
The removal of zinc from an alloy due to corrosion or heat |
|
Diaphragm |
A sheet of thin material designed to hold fluid pressure on one side only and deflect without damage. see also Membrane |
|
Diamagnetism |
The repulsion of a non-magnetic material from a magnet |
|
Diatomic (gas) |
A gas (mixture or pure) comprising only molecules of two atoms, such as nitrogen, oxygen, fluorine, chlorine, etc. |
|
Dirac's Constant |
ħ = h ÷ 2π = 1.05457207144921E-34 J.s |
|
Direct Current |
Direct current Steady-state current (and voltage) that does not vary in cycles (see AC). DC current is normally supplied by battery and used for low-power applications (<100W) such as mobile phones, computers and hand-held tools. DC generators are also used for DC power supply but much less frequently (than for AC power). |
|
Disorder |
A measure of randomness in the molecules that comprises a system (e.g. gas, liquid, solid, etc.) Randomness, and hence disorder, increases as molecules move further apart and/or move out of alignment, such as in crystaline arrangements, which occurs due to an increase in temperature and/or through decay. |
|
Distributed Load |
Also known as a line load, is applied (not necessarily equally) to a body along a line (not necessarily straight) and denoted as a load per unit length |
|
Down Quark |
One of the two types of quark that make the proton and the neutron and forms part of the first generation of matter The down quark is classified as a fermion mᵣ=8.538949767E-27g [4.79MeV/c²], Q=-⅓e, Iz=-½ |
|
Drag |
The magnetic field attraction between atoms and molecules in a fluid, slowing down relative movement. see technical help, state of matter and The True Atom |
|
Drag Coefficient |
A coefficient that represents the resistance between a fluid and a surface over which it is passing and with which it is in contact Such resistance is due to two conditions: Relatively smooth surfaces and low profiles will have a low drag coefficient (< 0.7) whilst rougher surfaces and high profiles will exhibit higher drag coefficients (> 0.7) Whilst there are certain circumstances where a drag coefficient can exceed 1.0, it is a rare occurrence see technical help, state of matter and The True Atom |
|
Draw |
The process of reducing the diameter of a filament (single or individual metal wire) by pulling it through a small die |
|
Duplex (stainless steel) |
A stainless steel containing approximately equal proportions of Ferrite and Martensite |
|
Dynamic Amplification Factor |
A factor by which a force (or weight) is multiplied in order to account for the dynamic nature of its condition e.g. F = mass x g x DAF |
|
Dynamic Pressure |
The pressure difference induced either side of a body travelling through a gas; In reality; see Aerodynamics |
|
E = m.c² |
Describes the potential energy (PE) in a proton-electron pair at the instant its particles unite to become a neutron. KE = ½.m.c² see E=mc² & Neutron and Laws of Motion |
|
Earth (properties) |
Diameter @ poles: 12713.504km see Earth's Properties, Earth's Magnetic Field and Earth's Atmosphere |
|
Eccentricity |
The ratio of the length of an ellipse's major axis (a) to its minor axis (b) The eccentricity of a circle = 0 see Elliptical Curves |
|
Elastic Moduli |
Describes the two and three-dimensional behaviour of materials under elastic deformation and includes: shear, bulk and Young's modulus along with Poisson's ratio |
|
Elastic Strain |
A material that returns to its pre-deformation dimensions when stress is released |
|
Elastic Stress |
A load per unit area that is less than the material's yield stress see also Elastic Strain |
|
Electrical Charge |
See Coulomb |
|
Electrical Energy |
The energy generated by electrical charge. It may be variously described, such as; RAMₚ = relative atomic mass of a proton; 1 gramme per mole (0.001 kg/mol) |
|
Electric Furnace |
The Electric Furnace is a process for melting steel. It uses high current electricity to melt the steel. It can be combined with the Bessemer Converter and Open Hearth methods by dipping charged electrodes into the melt. This process is capable of very high volume production and good quality control. It is also relatively inexpensive |
|
Electricity |
The movement or transfer of electrical charge, which occurs in both atomic particles and electro-magnetic energy. |
|
Electrode |
An electrical conductor (solid metal) that can transfer ions to or from an electrolyte |
|
Electrolyte |
A positive or negative electrically charged liquid that conducts electricity through ionisation. Seawater is a predominantly negatively charged electrolyte that will Corrode relatively positively charged metals (e.g. aluminium and zinc) and plate (coat) relatively negatively charged metals (e.g. carbon steel and tin) if immersed together. However, because water molecules are polar (they have both positive and negative probes), immersed steel will corrode in the absence of a donor material (e.g. aluminium or zinc) |
|
Electro-Magnetic Energy |
The helical radiation of alternating polar electro-magnetic field energy by proton-electron pairs. 
EME has no mass and travels at the speed of light in a straight-line unless deflected by magnetic charge; EME is trapped by orbiting electrons, which they convert to kinetic energy; Its wavelength, amplitude and frequency are all related thus: see Spectrum |
|
Electro-Motive Force |
The work done to move electrons by an electrical force (emf) through a material with electrical resistance. Whilst 'emf' and potential difference ('pd') are normally differentiated, they are essentially the same, as are their units of measurement (Volt). |
|
Electron |
A single indivisible packet of electro-magnetic charge that possesses intrinsic kinetic energy. mass = 9.1093897E-31 kg, radius = 1.45046059426276E-16 m see The Proton-Electron Pair |
|
Electron Affinity |
The energy required to remove an electron from a negative ion |
|
Electronegativity |
A tendency for collecting electrons These numbers (between 1 and 4), as defined by Pauling, are used to determine the contribution an element will make to an ionic or covalent bond. The higher the Electronegativity, the more electrons it will collect In reality, however, electronegativity is simply a measure of an atom's positive ionic strength; |
|
Electron Shell(s) |
The orbital path of an electron about its proton partner. All shells are full at all times. There are no shell valencies. see The True Atom |
|
Electron Shell(s) |
Shells are numbered 1 to 7 (in the periodic table) The numbers of electrons in each shell are as follows: Shell 1: s₂ = 2 In theory; after filling sub-shell f, shell number 5 should create another sub-shell but 'f' represents the limiting force-field All of which, is of course, fiction. |
|
Electron Volt |
The energy received by the charge of an electron due to the potential difference of 1 Volt see also Elementary Charge Unit |
|
Element |
A bar, beam, plate or member that constitutes part of a larger structure but which can be analysed individually |
|
Element |
see atomic element |
|
Elemental Matter |
A body of matter comprising atoms all of which have the same atomic number. |
|
Elementary Charge Unit |
The negative charge (-Q) carried by a single electron or the positive charge (+Q) carried by a single [lone] proton; i.e. a proton that is not part of proton-electron pair.) 1 elementary charge unit [Coulombs] = see UniQon |
|
Elementary Particle |
The fundamental particles of matter. Fermions |
|
Ellipse |

The circumference of a flat section cut at any angle through a right-circular cylinder where; x²/a² + y²/b² = 1 see Elliptical Curves |
|
Emission (EME) |

Electro-magnetic energy radiated by a single proton-electron pair. |
|
End Cap (force) |
A longitudinal tensile or compressive force in the wall of a pipe as a result of a variation in temperature and/or pressure see also Upheaval Buckling |
|
Energy |
Essentially the same as work with the same units Energy sources can be mechanical, electrical, chemical or thermal There are three principle forms of mechanical energy: There are four principle forms of thermodynamic energy: There are a number of atomic-nuclear energies, four of which are: |
|
Energy Moment |
Energy applied at a given distance from a datum. The modified version of Planck's constant (h') is an energy moment (J.m & ft.lbf.ft). |
|
Enthalpy |
The total energy of a system The units of measurement are J, Btu, W.s, N.m, lbf.ft, etc. see Thermodynamics |
|
Entropy |
The measure of disorder in a system defined by energy that cannot be converted into work Entropy is only a measure of disorder;it is not a measure of temperature temperature simply affects entropy To explain ... Entropy is the basic level of energy inherent in all molecules that is defined by the following formula: Temperature increases as heat energy is added to the above basic level. The temperature of any substance is defined by its gas constant (Rₐ) and limited by its heat capacity (cv). Therefore, as a substance gains or loses temperature (heat energy) its entropy will be affected due to its molecules moving further apart, thereby increasing disorder. So: Process formulas for a change in entropy such as: see Heat and Thermodynamics |
|
Epicycloid (curve) |

An epicycloid is a curve generated by a point on the circumference of a circle that is rolled around the outside of another circle without slipping. Special Case: The path generated by a point on the circumference of a rolling circle with the same radius as the stationary circle will be a 'Cardioid'. see also Cycloid, Trochoid and Hypocycloid |
|
Epitrochoid (curve) |
see Epicycloid |
|
Equilateral (triangle) |
A triangle with all three sides of equal length |
|
Equilibrium |
A state of constancy, stability and balance Equilibrium applies to mechanical, chemical, electrical and thermal systems that may be transferring energy but the exchange is constant, i.e. unchanging |
|
Equilibrium Diagram |
see also Phase Diagram |
|
Equivalent Stress |
Normally interchangeable with combined stress When differentiating between two different types of combined stress, CalQlata uses the following specific definitions: see also Stress |
|
Erosion |
The removal of matter by particles in a transient fluid. |
|
Escape Altitude |
The altitude at which gravitational acceleration of a body (e.g. planet, star, etc.) is equal to the centrifugal acceleration due to the orbital velocity (v²/r) of a satellite holding a constant position over the body This altitude for the earth is 35843425.4809m above sea level. see also Geosynchronous Orbit |
|
Euclidian Geometry |
A universal method of geometric calculation (developed by the Greek mathematician Euclid 323 BC - 285 BC) based upon a few basic rules. |
|
Eutectic |
A mixture of two metals that are completely soluble in a molten state but completely insoluble in a solid state. As the liquid cools to form a solid, alternate layers of each pure metal will form in the crystal(s) creating a laminated structure (like plywood). |
|
Eutectoid |
A mixture of two metals in a solid state that are completely soluble at a high temperature but completely insoluble at a lower temperature. As the material cools to form a different lattice structure (e.g. the transition of iron with 0.83% carbon from austenite above 723°C to pearlite below 723°C), alternate layers of each pure metal (or ferrite in steel) will form in the crystal(s) creating a laminated structure (like plywood). |
|
Exosphere |
Stratum of the earth's atmosphere that constitutes its outer limit (Ionosphere to 1000km) Contains neutrons and protons that have been detached by the sun's electromagnetic radiation Temperature increases exponentially with increasing altitude |
|
Explosion |
Sudden release of energy, that is usually chemical but it can also be pressure An explosion is always accompanied by an increase in heat and sometimes by deflagration. |
|
Fabric |
A structural material that comprises interwoven filaments the properties of which define those of the fabric. |
|
Face Centre Cubic |

Describes the lattice arrangement of atoms within a metal. The smallest crystal of this metal will contain fourteen closely packed atoms, eight of which are located at each corner of a cube and one in the middle of each face of the cube. |
|
Factor |
A multiplier, normally used to modify a calculated value to give a more representative or expected value, either for reasons of safety or to account for the 'unknown' (unreliable or missing data in the calculation). see also Coefficient |
|
Farad |
The unit of electrostatic capacitance that when charged by the potential difference of 1 volt carries a charge of 1 Coulomb. |
|
Faraday's Constant |
The magnitude of electric charge per mole of electrons: Original value: NA = 6.02214129E+23 /mole C-12 value: NA = 5.97538412973187E+23 /mole CalQlata uses the C-12 value as it provides greater accuracy when the associated 'NA' is applied to other constants. |
|
Fatigue |
The deterioration of a material due to repeated stress cycling |
|
Fatigue Life |
The total number of stress cycles (sum of all stress blocks) that represent the expected life of a material or mechanical component and above which damage or failure will occur |
|
Faxen/Oseen Correction Factor |
Correction factor for calculating the forces on a sphere falling through a fluid close to solid wall (e.g. in a tank or pipe). |
|
Fermion |
Any particle that obeys Fermi-Dirac statistics Fermions include sub-atomic particles; leptons and quarks and compound particles; neutrons and protons Fermions have half integer spin properties: ½, 1½, 2½, 3½, 4½, 5½, etc. see also Pauli's Exclusion Principle |
|
Ferrite |
Body centre cubic iron, which can absorb up to 0.006% carbon within its crystal structure at room temperature and up to a maximum of 0.03% carbon at 723°C (also called alpha iron) Pure ferrite has the following physical properties: see Carbon Steel |
|
Ferromagnetism |
Matter's ability to generate magnetism. |
|
Filament |
A single very thin wire or cord of any cross-sectional shape that normally forms part of a multi-filament construction, e.g. a strand see also Wire Rope |
|
Fire-Point |
A temperature above which a substance will give off sufficient vapour to maintain combustion This temperature is usually higher than the Flash-Point for the same substance see also Flash-Point |
|
Fission |
The splitting of an atomic element's neutrons into their component parts; a proton (alpha-particle) and an electron (beta-particle), releasing their stored energy Because neutrons are created in pairs, they revert to their component parts (a proton and an electron) in pairs. |
|
Fixed Support |
A support that prevents all six degrees of freedom |
|
Flammable |
Having the ability to catch fire under certain conditions see also Inflammable |
|
Flash-Point |
A temperature above which a vapour from a substance will combust if exposed to a spark or small flame It is usual to define this value on the basis of test using a Cleveland cup or Penskey-Martens apparatus This temperature is usually lower than the Fire-Point for the same substance |
|
Flexible |
The ability to deform significantly without breaking (the opposite of brittle). |
|
Flexural Modulus |
The deformation stress behaviour associated with polymers |
|
Fluid |
The term fluid is misleading as it encompasses two disparate states; viscous and gaseous. see State of Matter |
|
Focal Length |

The distance along the axis of a lens between a principal plane (at its axis) and its nearest focal point. see also Power (of a lens) |
|
Focal Point |

The theoretical point (F) of intersection between the axis of a Lens and an exiting light-ray that entered the lens parallel to its axis at a radial distance of almost zero. The primary focal point occurs at the front of a lens |
|
Force |
A load applied by an accelerating body (mass) on another body (mass), that may be stationary or in motion, thus changing their states of motion There are a number of specially applied forces, such as: Force = mass multiplied by acceleration (F = m.a) |
|
Force-Centre |
A mass (or body) maintaining a satellite in its orbit, e.g. see also Central Force Motion |
|
Free Cutting Steel |
Free cutting steel is plain carbon steel with good machinability Plain carbon steel with more than 0.2% carbon is relatively difficult to machine because of its hardness and its embedded manganese increases this hardness. Sulphur is added to plain carbon steel to improve machinability, which it does by combining with the manganese to create manganese sulphide. This compound ensures clean chip separation without brittle fracture during machining. |
|
Free Support |
A support that allows all six degrees of freedom |
|
Frequency (ƒ) |
The number of times a cyclic event is completed in a given time period. A frequency of a number of cycles each 'second' is measured in 'Hz'. The higher the frequency (i.e. the shorter the wavelength) the greater the energy in the wave The natural frequency (ƒⁿ) of an object is the frequency at which it can regenerate a complete cycle itself (free oscillation) with minimal external assistance and is dependent upon the elasticity and inertia in the system. The natural frequency (ƒⁿ) of an object is the frequency at which it can regenerate a complete cycle itself (free oscillation) with minimal external assistance and is dependent upon the elasticity and inertia in the system. The relationship between frequency and wavelength for electro-magnetic energy (e.g. light): |
|
Freeze |
|
|
Friction |
Friction is the resistance to relative movement between two surfaces see also Stiction and Coefficient of Friction |
|
Frustum |
A pyramid truncated by a flat plane normal to its axis |
|
Fulcrum |
The point about which a lever or moment couple rotates Also called a pivot point |
|
Fusion |
The joining of elements (e.g. two hydrogen atoms to make one helium atom) by pushing the nucleus of one atom inside the electron shells of another. Fusion only occurs naturally inside cold bodies of sufficient mass to generate the necessary internal pressures; i.e. galactic force-centres, the great attractor and the ultimate body. see Core Pressure |
|
Galactic Force-Centre |
A force-centre at the focal point of a galactic system. see The Universe |
|
Galileo's |
Inertia: Every object persists in its state of rest, or uniform motion (in a straight line); unless, it is compelled to change that state, by forces impressed on it (Newton's first law) Falling Objects: The distance travelled by a falling body is directly proportional to the square of the time it takes to fall Uniform motion: d ∝ t Uniform Acceleration: v ∝ t Parabolic Curve: A projectile that with a uniform horizontal and a naturally accelerated vertical motion describes a path which is a semi-parabola (i.e. half of a full parabolic curve) Terminal Velocity: A body falling from a very considerable height will reach a velocity that will remain constant due to frictional resistance from the surrounding air (see CalQlata's Fluid Forces calculator) Frame of Reference: Any two observers moving at constant speed and direction with respect to one another will obtain the same results for all mechanical experiments see Laws of Motion |
|
Galvanic Corrosion |
Describes the form of corrosion whereby a sacrificial metal (anode) will lose material (protons) to another metal (cathode) Whilst this phenomenon normally occurs due to both metals being immersed (or wetted) by a common liquid (an electrolyte) facilitating the electrical transfer of positively charged ions from one to the another, it can occur if both metals are joined by any similarly capable medium. The electrical property responsible for this phenomenon in metals is Electronegativity, which indicates their relative nobility |
|
Gamma Iron |
Pure iron (> 1,183 K) with a face centre cubic crystal structure holds up to 2% carbon in solution |
|
Gamma Radiation |
Very short-wave electro-magnetic energy released as a result of neutron decay, the proton of which remains trapped within the atom's nucleus. No particles are emitted as a result of Gamma radiation. λ < 1.77056263481047E-14m to 1.0E-11m |
|
Gas |
A substance in a state whereby the electrical repulsive forces between all its atomic and molecular protons is greater than their magnetic [attractive] forces. The natural state for any gas is to equalise its 'same-element' pressures filling its container, which includes the effect of gravity. All same-element atoms (and molecules) will repel according to their lattice-structures. see also Liquid, Solid and Dalton's Law |
|
Gas Constant |
The energy required to raise the temperature of an ideal gas by one degree Universal (ideal) constant for all gases {per mole}: Specific (mass) gas constant {per unit mass}: Gas constant {for total mass of gas}: Gas constants for sub-atomic particles (historic values): see Heat, Thermodynamics and Steam (properties) |
|
Gas:Oil Ratio |
The volumetric (e.g. ft³ or m³) ratio of oil to gas at 1 atmosphere and 60°F |
|
Gas Planet |
A planet that has collected sufficient satellite mass to the raise its internal [frictional] temperature to melt its surface matter. see Planetary Spin |
|
Gas Transition Temperature |
The temperature at which the proton electrical repulsion forces (Fₑ) and magnetic field attraction forces (Fₑ) are equal. In other words; see State of Matter and Density & Temoerature |
|
Gauge Boson |
Bosonic particles that act as carriers of the fundamental forces of nature |
|
General Relativity |
A theory that defines the motion of objects based upon the deformation of space and gravity, see Relativity is Dead |
|
Generator |
A generator uses magnets and coils of copper wire to convert rotary motion into DC or AC electricity. see also Alternator and Motor |
|
Geosynchronous Orbit |
The altitude where the gravitational acceleration of a planet/star equals the centrifugal acceleration on an orbitting satellite that remains continuously over exactly the same spot on the planet. R = ³√(G.m/ω²) Increased speed at this altitude will result in the centrifugal acceleration being greater than gravitational acceleration causing the satellite to drift out into space. see also Escape Altitude |
|
Gluon |
A strong force-carrying gauge boson or messenger particle Gluons are elementary expressions of quark interaction and indirectly involved with the binding of protons and neutrons together in atomic nuclei There are 8 types (or colour) of gluon, any combination of two from the following colours: A gluon is a Gauge Boson m=0g, Ø≈10ˉ¹⁵m, lifetime>10²⁹yrs, Q=0, Iz=1 |
|
Golden Ratio |
A universal ratio found in nature and mathematics which was first used by Phidias (500 BC - 432 BC; after whom the value is named) Φ-1 = 1/Φ |
|
Grain (crystal) |
A complete single crystal of similar atoms in the form of a naturally occurring lattice structure A grain of any material can also contain atoms of a different type but these atoms must be small enough to fit into the gaps (spaces) within the crystalline lattice structure of the granulated material Grain growth produces in large (coarse) grain structures resulting brittle metals |
|
Gravitational Acceleration |
Gravitational acceleration is the straight-line potential acceleration induced by a mass: Where it is necessary for CalQlata to impose a default value for 'g' at the surface of the earth in any of its calculators (e.g. UniQon), the value used at sealevel is; 9.80663139027614 m/s² (which happens to be at latitude 45.5° {Milan or Minneapolis}). a) ISO states that; 1lb (force) = 4.448222N (exact) b) ISO also states that; 1lb (mass) = 0.45359327kg (exact) c) The above defaulted value for 'g' appears if you d) CalQlata default's to the above value for consistency between its calculators and this website The imperial equivalent for the above 'exact' value for 'g' is 32.173987500906ft/s² CalQlata's UniQon-technical help includes a facility for calculating an accurate value for 'g' (at your latitude) should you require one. |
|
Gravitational Constant |
A universal constant that defines the gravitational attraction (force) between two or more bodies G = aₒ.c² / mᵤ = 6.67359232004332E-11 m³/kg/s² © G = φ.k.e² / mₑ.mp The Imperial equivalent of which is; see also Gravitational Constant |
|
Gravitational Constant |
A dimensionless conversion constant for gravitational acceleration used in calculations dependent upon their units |
|
Gravitational Energy |
The attractive potential energy exerted by a mass due to the combined non-polar magnetism in all its atomic particles: see also Gravity |
|
Gravitational Force |
The non-polar magnetic attraction acting between two or more bodies that is a function of the magnetic charge in all their atomic particles: |
|
Graviton |
Is a hypothetical gravitational force carrying gauge boson It is not yet known if it exists m=0g (at rest), Ø≈0m, lifetime=∞, Q=0, Iz=2 |
|
Gravity |
The attraction between all the atomic particles in all matter due to their magnetic charges. |
|
Great Attractor |
The body of matter left over from after the last 'Big-Bang' that acts as a non-orbital force-centre slowing down the outward travel of galactic force-centres. see The Universe |
|
Guided Support |
A support that allows four degrees of freedom; lateral movement in both planes, axial movement and axial twist |
|
Hades |
The force-centre at the heart of our Milky Way galaxy has the following properties: see Dark Matter and The Universe |
|
Hadron |
Is a bound state of quarks, which includes protons and neutrons |
|
Half Life |
The time taken for a substance of same-elemental atoms to lose two neutrons from half of those with a neutronic ratio greater than 1. see Carbon-Dating |
|
Head (liquids) |
The height of the surface of a liquid above a specified datum |
|
Heat |
The electro-magnetic energy radiated by proton-electron pairs; Conduction is the transfer of energy between atoms in both viscous and gaseous matter. Convection is the repositioning of gaseous atoms and molecules in order to balance their proton electrical repulsion forces (Fₑ) and the magnetic (gravitational) attraction forces (Fₘ) to minimise pressure. |
|
Heat Capacity |
The quantity of heat energy that can be absorbed by a given mass of substance per unit temperature. heat capacity = mass {kg} x specific heat c-capacity {J / K.kg} Heat Capacity of a proton-electron pair: The units of measurement are J/K, Btu/°R, W.s/K, N.m/K, lbf.ft/°R, etc. see Heat |
|
Heat Energy |
The quantity of heat (energy) in a system at atmospheric pressure Q = Ṯ.Cp The units of measurement are J, Btu, W.s, N.m, lbf.ft, etc. see Heat and Thermodynamics |
|
Heat Transfer Coefficient |
The rate at which heat flows through a material or substance of specified thickness. |
|
Heat Transfer Constants |
The rate at which electro-magnetic energy is converted into electron orbital velocity (and radius): Orbital Velocity (v): Orbital Radius (R): |
|
Heat Transfer Constant |
Temperature constant used to define specific heat capacity of an atom: |
|
Heat Transfer Rate |
The rate at which heat flows from a material or substance of unit volume. |
|
Heave (vessels) |

The linear movement of a vessel in the direction of the vertical ('z') axis Whilst this term generally applies to the movement of ships, it is also used to define the movement of any object subject to the same degrees of freedom; e.g. aircraft and spacecraft see Right-Hand Rule for positive and negative directions of this movement |
|
Helical Gear |
A gear wheel, with teeth cut into its circumference in the form of a helix, that mates with another helical gear wheel The tooth profile is similar to that of a spur gear tooth The centres of rotation of two mating helical gear wheels are not necessarily parallel |
|
Henry |
The unit of mutual inductance such that... It can also be described as the rate of change current induced in an electrical circuit (C/s²) The unit Henry becomes energy when multiplied by electrical current squared (I²); E = kg.m²/C² . (C/s)² |
|
Heisenberg's Uncertainty Principle |
|
|
Hexagonal Close Packed |

Describes the lattice arrangement of atoms within a metal. The smallest crystal of this metal will contain twelve closely packed atoms, all of which are located at each corner of an hexagonal prism. This is the most closely packed of all the lattice structures. |
|
Higgs Particle |
The Higgs particle is a very unstable boson with no spin, electric charge or colour charge that almost immediately decays into other particles. It is a carrier of the Higgs field, which is supposed to give everything a mass component and without which all particles would simply be energy packets flying about at the speed of light. m≈2.246153801E-25g [≈126GeV/c²], Iz=0 |
|
Hooke's Law |
States that stress and consequent strain obey a linear relationship and that dimensional recovery is total when the material is relaxed This law applies to any elastically deforming body (e.g. a spring, a steel body, rubber band, etc.) all of which have a unique spring constant ('k') that defines the force needed to deform the body by one unit of length see also Young's modulus |
|
Hoop Stress |
Primary circumferential stress in a pipe or cylinder |
|
Hydrocarbons |
Molecules comprising only hydrogen and carbon atoms Predominantly organic fluids (liquids and gases) originating from degraded and compressed animal and/or vegetable matter Includes; glycol, glycerine, benzene, propane, methane, ethylene, toluene, etc. |
|
Hydrodynamic |
Associated with the movement of water. Whilst the term 'hydro' refers specifically to water, hydrodynamic references may be applied to the movement of any liquid. |
|
Hydrogen (atom) |
Hydrogen is a collective term for four elements, all of which possess the same atomic number; Whilst 'D' and 'T' can (and do) exist in viscous form (e.g. in heavy water), it may be that at a sufficiently low temperature, the magnetic field generated by 'H' will be greater than the positive electrical charge in its proton, allowing it to exist in viscous form. |
|
Hyperbola |

The perimeter of any vertical plane through two inverted right-circular cones where; x²/a² - y²/b² = 1 see Elliptical Curves |
|
Hyper-Eutectoid |
A mixture of two metals that exist above their eutectoid state For example, an hyper-eutectoid steel is a mixture of pearlite and cementite; i.e. solid iron with > 0.83% carbon below 723°C |
|
Hypersonic |
Generally refers to a velocity more than twice Sonic; see also Subsonic, Supersonic, Transonic |
|
Hypocycloid (curve) |

A hypocycloid is a curve generated by a point on the circumference of a circle that is rolled around the inside of another circle without slipping. Special Cases: see also Trochoid, Epicycloid and Cycloid |
|
Hypo-Eutectoid |
A mixture of two metals that exist below their eutectoid state For example, an hypo-eutectoid steel is a mixture of pearlite and ferrite; i.e. solid iron with < 0.83% carbon below 723°C |
|
Hypotenuse |
The side opposite the 90° angle of a right-angle triangle |
|
Hypotrochoid (curve) |
see Hypocycloid |
|
Hysteresis |

A loop showing a delay in the recovery of one property that varies as a result of varying another. A complete hysteresis loop is one where recovery catches up in each cycle (as shown in diagram). |
|
Ideal Gas |
A gas, the molecules of which, are of negligible size and do not interact chemically with each other. For example: nitrogen, argon, carbon dioxide, oxygen (O₂) and air are all ideal gases Also called a perfect gas |
|
Ideal Gas Law |
The mathematical relationship between the pressure, volume and temperature of a gas molecule: p.V = n.Ri.Ṯ see also PVRT |
|
Impedance |
Impedance is the total electrical resistance in a conductor Impedance² = Reactance² + Resistance² |
|
Inductance (L) |

Inductance (the unit of measurement is the 'henry') is the resistance generated by the induced (or 'back') emf (electro-motive force), which opposes the applied emf and therefore retards the growth of an AC current Current lags voltage |
|
Induction |
The transmission of electrical current between disconnected conductors via a magnetic field. Note: a current passed through a conductor will generate a surrounding magnetic field, & a magnetic field will induce a current in an encircled conductor. |
|
Inertia |
A body's resistance to movement due to the influence of the magnetic force-field radiated by the magnetic charge in its atomic particles. Its units of measurement are the same as those of mass, e.g. kg, lb, etc. |
|
Inertia Coefficient |
A coefficient that represents the 'Added Mass' plus the mass of fluid displaced by the body itself Also known as Virtual Mass |
|
Inflammable |
In its strictest sense this means the inability to catch fire under any conditions, however, it is frequently used as an alternative for 'Flammable' see also Flammable |
|
Inorganic |
Elements that cannot intrinsically self-replicate. They generally (but not only) exclude carbon molecular chains. |
|
Intensity (EME) |
The number of EME emissions per unit area. |
|
Intercalate |
The accommodation of a body between other bodies. |
|
Internal Energy |
The energy in a system that has not been supplied from its surroundings. In a static system: U = Ṯ.Cv The units of measurement are J, Btu, etc. see Heat and Thermodynamics |
|
Inverse |
Mathematics: the opposite polarity of the same value. |
|
Involute (curve) |
An involute is the curve generated by unwinding (straightening out) the arc (or circumference) of a curved shape such as a circle. For example: the end of a cotton thread unwound from a reel and pulled tight (straight) will describe a curve of ever-increasing radius as the unwound length of thread gets longer. 
The figure shows the involute of a circle, which can, for example, be represented by the path generated by the end of the a thread as it is unwound from a reel. The circumferential length of arc ('A-O') is equal to the straightened out (unwound and tightened) length 'A-P'. The radius of the involute curve at any point is equal to the arc length of the circumference unwound. For example; in the figure, the radius of the curve at 'P' is equal to the straight length 'A-P', which is also equal to the curved length 'A-O'. Involute curves are almost always used for the profile of gear teeth for the following reasons: |
|
Ion |
The number of orbiting electrons in an atomic element relative to the number of protons in its nucleus. A positive Ion has fewer electrons than protons. A negative Ion has more electrons than protons. |
|
Ionic Bond |
Historic explanation: The greater the electrical polarity of each atom the greater the strength of the bond. Ionic bonds are generally stronger than covalent bonds. But bonds such as those in diamond (carbon) prove the exception is also true. However, there are no valencies in atomic electron shell's, so the above explanation is hypothetical. |
|
Ionisation Energy |
Historic explanation: In reality, however, it refers to the potential energy (Joules) required to pull (separtate) an atomic electron from it nucleic proton partner. |
|
Ionosphere |
Stratum of the earth's atmosphere that contains only atoms and light ions of nitrogen and oxygen (Mesosphere to 600km) Temperature increases with increasing altitude |
|
Isentropic |
A process during which no change in entropy occurs see Thermodynamics |
|
Isobaric |
A process during which there is no change in pressure Isobaric refers only to the pressure properties of a process, it is therefore possible for a process to be isobaric and isochoric and/or isothermal see Thermodynamics |
|
Isochoric |
A process during which there is no change in volume Isochoric refers only to the volume properties of a process, it is therefore possible for a process to be isochoric and isobaric and/or isothermal see Thermodynamics |
|
Isosceles (triangle) |
A triangle with two of its sides of equal length and therefore, two equal angles |
|
Isothermal |
A process during which there is no change in temperature Isothermal refers only to the temperature properties of a process, it is therefore possible for a process to be isothermal and isobaric and/or isochoric see Thermodynamics |
|
Isotope |
Defined by the number of neutrons, relative to the number of protons, in the nucleus of an atomic element For example: Uranium 238 (U²³⁸) is an Isotope of Uranium (there are also 235, 236, etc. Isotopes of Uranium) but U²³⁸ is the most common |
|
Isotropic |
A description of material proproperties that do not vary with direction, for example; |
|
Independent Wire Rope Centre |
A multi-stranded wire rope in which the core is an individual strand and not a fiber or polymer or single filament |
|
Jarno Taper |
A conical shaft and mating sleeve for machine-part self-holding applications that optimises assembly load, holding capacity and easy release (without damage). The tapered shaft is normally driven by an End-Tang (or Key) or a Longitudinal Key. You can have any size you like, but each taper is described dimensionally as follows, for example: Taper No. 7 Taper No. 12.35 All tapers have a slope of 0.6 inches per foot (2.86419236832929°) The Jarno Taper is almost a direct alternative to the Morse Taper and used for similar applications to the Brown & Sharpe Taper |
|
Joule |
A measure of energy named after James Prescott Joule, who developed the theory of heat 1 Joule is the amount of energy expended by moving ... |
|
Kepler's |
1ˢᵗ An orbiting body follows the path of an ellipse 2ⁿᵈ Any two (or more) equal areas of elliptical orbits will be swept by equal periods of time 3ʳᵈ The square of the period of motion is proportional to the cube of the orbital semi-major axis see CalQlata's elliptical curves calculator and Laws of Motion |
|
KERS |
Kinetic energy recovery system. The formula for kinetic energy is ½.m.v² It could be said, however, that the process of deceleration is the 'shedding' of velocity, and it is this shed velocity (kinetic energy) that is recovered. |
|
Keulegan-Carpenter Number |
Flow parameter associated with the drag of a fluid over an object. K = Uₘ.t / D see Vortex Shedding |
|
Kinetic Energy |
Energy possessed by a moving body Linear: Uᴷ = ½mv² ÷ gc Rotational: Uᴷ = ½Iω² ÷ gc Where: |
|
Laminar Flow |
A fluid that flows perfectly smoothly generating no disturbance or mixing of adjacent layers |
|
Lang Lay |
A particular type of helical lay pattern in a wire rope where the filaments are wrapped within each strand in the same helical direction as the strands are wrapped within the wire rope. In a manufactured wire rope, the filaments run at an angle (diagonal) to the long axis of the wire rope (see image).  You can have 'right Lang lay' or 'left Lang lay' configurations: Lang lay wire ropes provide excellent wear resistance when used with a sheave (or pulley) due to their smoother (flatter) surface and high bending fatigue resistance but suffer from birdcaging if ill used. They are generally used for specialist applications where repeated bending and wear resistance is an issue but structural stability is not. see also Regular Lay |
|
Latent Heat |
|
|
Lateral |
see Latitudinal |
|
Lateral Stress |
Primary stress at right-angles to the axis of a component |
|
Latitude |
The horizontal grid-lines that divide the surface of the earth into 180 unequal spherical segment zones (slices) The vertical distance between each gridline at the equator = 111319.8922m |
|
Latitudinal |
1-D distance or movement across the width of a body or shape; normal to its longitudinal line or axis. For a sphere; this refers to the shortest distance between two points over its surface; normal to its longitudinal lines. see also Longitudinal |
|
Lattice Structure |
General term referring to the structural arrangement of 'same-element' atoms in their lowest energy state, which applies to both their viscous and their gaseous conditions. The lattice structure of elemental matter is a replication of the structural arrangement of its element's nucleus. see also Cubic, Body Centre Cubic, Face Centre Cubic, Hexagonal Close Packed, Rhombic and Tetra-Hedra |
|
Latus Rectum |

The width of an elliptical curve (2p) at its focus (F) Also called the parameter of the curve |
|
Lens |

A device that converges electromagnetic waves (radiation) at a focal point# A lens may converge or diverge the radiation dependent upon the path of the light-ray in relation to the convex or concave nature of the lens faces. The front of the lens is the face at which the radiation enters the lens # The generally accepted position of the focal point of a spherical lens is theoretical (see Focal Length). |
|
Lepton |
A family of sub-atomic particles within the fermion group There are 6 leptons (Iz isospin quantum number); |
|
Lift Coefficient |
A coefficient used to define the lateral force (or lift) developed in a beam or structure as a result of a fluid passing along or across it at 90° to the direction of lift This coefficient varies between 0 and 4, and is dependent upon the Keulegan-Carpenter number, |
|
Light (visible) |
The range of electro-magnetic energy visible to the animal kingdom: infra-red > ultra-violet: Humans can see the visible range: The speed of light (c) in free-space (a vacuum) is defined as: |
|
Linear |
The shortest path between two points. Sometimes referred to as a vector or straight-line. |
|
Linear Expansivity |
|
|
Linear Acceleration |
The acceleration of a point travelling in a straight line see also Angular Acceleration and Rotational Acceleration |
|
Linear Velocity |
The velocity of a point travelling in a straight line see also Tangential Velocity |
|
Liquid |
Liquidity is not a genuine state of Matter. It is simply a measure of the viscosity of a solid; that which cannot hold its shape under gravitational energy. The liquid/solid transition temperature of matter is inversely proportional to gravitational energy. In other words, the melting temperature of, say, iron, will be greater on the surface of our moon than it is on the surface of our planet. # see State of Matter |
|
Locus |
The path generated by a point following a mathematical equation. for example: the locus of a point 'x,y' following the equation: |
|
Lodestone |
Naturally magnetic magnetite (Fe₃O₄ + Fe₂O₃). It is created in the earth's mantle via the magnetic field generated by the relative spin in the earth's core and mantle matter. Fe₃O₄ + Fe₂O₃ is uniquely suited to acquire and retain natural magnetism. see Magnetism, Magnetic Field and Planetary Spin |
|
Logarithm |
The logarithm value of a number see Logs and Trig-technical help |
|
Longitude |
The vertical (pole to pole) grid-lines that divide the surface of the earth into 360 equal spherical wedges (1°) The horizontal distance between each gridline at the equator = 111319.8922m |
|
Longitudinal |
1-D distance or movement along the length (long-axis) of a body or shape. For a sphere; this refers to the shortest distance over its surface between its poles. see also Latitudinal |
|
Longitudinal Stress |
Primary stress parallel with the axis of a component |
|
Lorentz's Magnetic Force |
Lorentz defined the force between two magnets thus: His formula actually distils down to: |
|
Mach [number] |
Ernst Mach's ratio of the velocity of a fluid and that of a sound passing through it. see Fluid Numbers |
|
Magnetic Charge |
The non-polar magnetic charge in all atomic particles that we currently refer to as mass (kg), accrues and attracts all other atomic particles; it does not repel. All mass comprises atomic particles that together radiate a constant collective force field throughout the universe. This force is distributed over the surface area at any radial distance 'R'. Because it accrues, this field, which is what we today refer to as inertia, means that Isaac Newton's and William Gilbert's field force formula includes the product of both attracting charges (masses) in full: F = G.m₁.m₂/R². The magnetic charges in the earth (m = 5.95786303763713E+24 kg) can be used to calculate gravitational acceleration at its surface like this:
see also Magnetic Field |
|
Magnetic Charge Constant |
Joseph Henry's magnetic field generated by the proton-electron pair at its neutronic condition: Its units of measurement (kg.m/C²) are occasionally referred to as the Henry (H) see also Permeability and Magnetic Field |
|
Magnetic Field |
The general formula for Joseph Henry's magnetic field at any given temperature {kg.m/C²}, occasionally referred to as Permeability: Its units of measurement (kg.m/C²) are occasionally referred to as the Henry (H) see Magnetism |
|
Magnetic Flux |
The magnetic equivalence of electrical current. Where: Φ = mmf/Reluctance. see magnetism |
|
Magnetism |
The attraction between metals due to the alignment and proximity of the magnetic charge in their atomic particles. Some magnetic materials are listed below: |
|
Magnetic Quantum Number |
The third in a set of quantum numbers that describes the energy level of the electron The magnetic quantum number of an electron can be a positive or negative integer: i.e. -5, -4, -3, -2, -1, 0, 1, 2, 3, 4, 5, etc. The magnetic quantum number must be different for each electron in the same shell with the same azimuthal and spin quantum numbers |
|
Magneto-Motive Force |
The energy needed to extract a magnetic charge from a proton-elecron pair. see magnetism |
|
Marageing |
An ageing heat treatment procedure used to harden, strengthen & toughen certain alloys (most alloys do not respond to this process). The age hardening process is performed as follows: A marageing alloy can therefore be machined or formed easily and subsequently strengthened with age over a very short period. The word originates from 'mar' for martensite (the steel structure) and 'ageing' to describe the process see Stainless Steels |
|
Martensite |
Super saturated austenite created by rapid cooling (quenching). Carbon exists in sharp clumps between the grains of iron making it hard and brittle. Tempering after quenching allows the excess carbon to form smaller spheroids and redistribute itself reducing hardness and brittleness |
|
Mass |
The constant non-polar magnetic charge in all atomic particles, the magnitude of which accrues collectively. All magnetic charges radiate a constant magnetic force-field throughout the universe. Its units of measurements are; kg, lbs, etc. For any given body, this property remains constant irrespective of liquefaction, dissection and location (in the universe) see also Inertia |
|
Matter |
A collective expression for all forms of substance, including both viscous and gaseous. see State of Matter |
|
Maximum Metal Condition |
The condition of at least two assembled components that when machined within required dimensional tolerances results in the removal of the least material Whilst convention does not differentiate between minimum and maximum metal conditions when using the symbol 'MMC', CalQlata always uses MMC+ for this condition see Symbols |
|
Mechanical Equivalent of Heat |
A conversion factor for heat energy and work energy Calculations using metric units; Calculations using Imperial units; |
|
Median |
A straight line that joins a vertex with the centre of the opposite side |
|
Medium |
The substance of an environment, e.g.; |
|
Melt |
The viscous state of matter under which it can no longer maintain its shape. In fact, a molten state is simply a very low viscosity condition that will occur at different temperatures dependent upon location, gravitational acceleration and ambient pressure; |
|
Member |
A longitudinal component such as a structural element, rod, bar or beam |
|
Membrane |
A large plate or sheet of thin material than can comprise any natural or manufactured matter see also diaphragm |
|
Membrane Stress |
Tensile or bending stress in the flat plane of a membrane see also Membrane |
|
Meson |
A pair of sub-atomic particles comprising one quark and one anti-quark (part of the Hadron particle family) |
|
Mesosphere |
The ceiling for 99.95% of nitrogen, the earth's most abundant atmospheric gas (Stratosphere to 80km) Stratum of the earth's atmosphere that comprises the ceiling of normal atmospheric gases (see Ionosphere) Temperature reduces with increasing altitude |
|
Metacentre |

The point at which a vertical line passing through the centre of buoyancy of a slightly tilted vessel meets a line through its centre of gravity (see image). Metacentric height: The vessel is stable if the metacentre is above the centre of gravity and unstable if it is below. A vessel's stability is defined by the metacentric height. |
|
Metallic Bonds |
Metallic elements lose electrons freely generating a cloud or sea of electrons in which positively charged metal atoms (cations) are held together. Metallic bonds tend to be the easiest to form and also the easiest to break, making metals the most versatile of the elements. |
|
Microstate |
The volumetric, pressure and heat energy states of atomic particles governed by the relationship: cᵨ.Ln(Ṯ).RAM = Rᵢ.Ln(N) volumetric: Nᵥ = cᵥ/Rₐ Minimum values: see Heat |
|
Microwaves |
Long wave electromagnetic radiation that falls between infra-red light and radio-waves Ranges from: |
|
Minimum Metal Condition |
The condition of at least two assembled components that when machined within required dimensional tolerances results in the removal of the most material Whilst convention does not differentiate between minimum and maximum metal conditions when using the symbol 'MMC', CalQlata always uses MMC- for this condition |
|
Mode |

The beam length deflected between two nodes see also Amplitude |
|
Modulus of Elasticity |
The measure of a substance's ability to resist deformation under pressure (or stress) and return to its original shape when the pressure (or stress) is released. the general formula for this property is; see also bulk modulus, elastic modulus and rigidity modulus |
|
Modulus of Rigidity |
The measure of a substance's ability to resist deformation under a shear force and return to its original shape when the shear force is released. the general formula for this property is; see also Shear Stress |
|
Modulus of Shear |
|
|
Molarity |
|
|
Mole |
The mass of a material that contains Avogadro's number of particles (atoms, molecules, etc.) For example; To calculate the number of moles in any mass of matter: To calculate the mass of a number of moles of matter: see also Avogadro's number |
|
Molecular Density |
The mass density (g/m³) of a substance divided by its relative atomic mass (g/mole) ρᴹ = ρ ÷ RAM (moles/m³) see also Molecular Weight |
|
Molecular Weight |
The sum of relative atomic masses comprising a molecule This calculation can also be applied to a mixture of gases (i.e. not a molecule) in order to specify its molecular weight |
|
Molecule |
The smallest part of a substance that exhibits its chemical properties. A molecule is at least two electrically charged ions joined together via ionic or covalent bonding. |
|
Molten |
A condition of viscous matter whereby its resistance to shear is insufficient to maintain its shape under prevalent gravitational acceleration. |
|
Moment |
An applied load or centre of area a given distance from a datum Also known as torque or rotational moment or bending moment or area moment |
|
Moment Couple |

Two parallel opposing forces applied a specified distance from each other |
|
Moment of Inertia |
Also (incorrectly) known as second moment of area, describes the mass of a body about its neutral axis That is, the sum of all the particle masses multiplied by the square of their distances from the neutral axis (radii of gyration) I = A.ɍ² |
|
Moment of Inertia |
Inertia possessed by a body rotating about a central axis I̊ = ⅖.m.r² |
|
Momentum |
A quantity of straight-line motion of a body equal to its mass multiplied by its velocity; Also a measure of force (m.a) multiplied by its period of application (in seconds); |
|
Monoclinic |
The arrangement of atoms within the shape of a single metal crystal. This crystal shape is a single atom located as close as possible to its neighbour. |
|
Monatomic (gas) |
A gas (mixture or pure) comprising only single atoms, such as argon, radon, helium, neon, etc. |
|
Morse Taper |
A conical shaft and mating sleeve for machine-part self-holding applications that optimises assembly load, holding capacity and easy release (without damage). The tapered shaft is normally driven by an End-Tang (or Key) or a Longitudinal Key. Different size numbers (0 to 7) relate to different engaged lengths (1-15/16" to 9-1/2") Morse's nominal included angle is 2.86419236832929° The optimum angle for a coefficient of friction of 0.8 for clean, dry, ground, carbon steel surfaces is 2.9745503204919° The Morse Taper is a direct alternative to the Jarno Taper and used for similar applications to the Brown & Sharpe Taper |
|
Most Damaging Stress-Block |
The stress-block that lies closest to the S-N curve, within a group of stress-blocks that together constitute the fatigue life of a material or mechanical component |
|
Motor |
A motor uses magnets and coils of copper wire to convert AC or DC electricity into rotary motion. see also Alternator and Motor |
|
Multi-Phase |
The flow of more than one category of fluid. Normally applied to the flow of fluids through a pipe in the petroleum industry; see also Single-Phase |
|
Muon |
An elementary particle similar to the electron (also with a negative electric charge) but much heavier A muon is classified as a lepton m=1.884273466E-25g [105.7MeV/c²], lifetime=2.2μs |
|
Natural Frequency |
see Frequency |
|
Natural Period |
see Frequency |
|
Nappe (conic) |
One of two sheets on either side of the vertex forming a cone |
|
Natural Logarithm |
The logarithm value of a number, the base of which is 2.71828182845905 see Logs & Trig-technical help see also Logarithm |
|
Nature |
The intrinsic behaviour, extinction, creation and evolution of [universal] inorganic and organic matter. |
|
Necking |
The reduction in cross-sectional area of viscous matter exposed to a tensile stress between yield and UTS. |
|
Neutral Axis |
The plane through a bending body in which no tension or compression occurs Note: tension occurs in the material on convex side of the neutral axis and compression occurs in the material on concave side |
|
Neutrino |
An elementary particle with minuscule but nonzero mass that often travels close to the speed of light, that lacks an electric charge and is able to pass through ordinary matter almost undisturbed and are thus extremely difficult to detect A neutrino is classified as a lepton Neutrinos are created as a result of certain types of radioactive decay or nuclear reactions such as those that take place in the Sun, in nuclear reactors, or when cosmic rays hit atoms. There are three types, or "flavours", of neutrinos: electron neutrinos, muon neutrinos and tauon neutrinos; each type also has an antimatter partner, called an anti-neutrino. Interactions involving neutrinos are generally mediated by the weak force |
|
Neutron |
An electrically neutral atomic particle that comprises a proton and an electron that united at the neutronic temperature. This event occurs when the electron is orbiting at the neutronic radius, which is achieved when it reaches light-speed (PE=mc²). Neutrons are only created within, and can only exist within, an atomic nucleus. And they are only released from their nucleus after they have been split into their component parts. mass; mₙ = mₚ + mₑ = 1.6735325768E-27 kg see The Neutron |
|
Neutron Decay |
The revertion of neutrons to alpha and beta particles. This event occurs in all atoms with a neutronic ratio greater than 1.0 see The Neutron |
|
Neutron Energy |
Every neutron holds the same potential, kinetic and spin energies its proton-electron pair was generating when it was created: Potential Energy; PE = -mₑ.c² = -8.18711122262534E-14 J see The Neutron |
|
Neutronic Radius |
The orbital radius that an electron partner will achieve when it reaches the speed of light due to the neutronic temperature, i.e. when magnetic field attraction energy exceeds electrical charge repulsion energy in an atomic proton-electron pair. |
|
Neutronic Ratio |
The ratio of neutrons to protons in an atomic nucleus; ψ = RAM/Z-1 |
|
Neutronic Temperature |
The temperature required to drive an orbiting electron at the speed of light and at the neutronic radius, see The Neutron |
|
Newton's Atom |
Newton's atomic values are calculated below: see Laws of Motion |
|
Newton's |
The potential (gravitational) force between any two bodies of mass (m₁ & m₂) separated by distance 'R'. F = G.m₁.m₂ / R²) see Laws of Motion |
|
Newton's |
1ˢᵗ Every mass retains its momentum (including rest) until a force acts upon it (Galileo's law of inertia) 2ⁿᵈ A change in momentum is proportional to, and acts in the direction of, the applied force 3ʳᵈ To every action there is an equal and opposite reaction see Laws of Motion |
|
Newton's Mass |
Unit mass of ultimate density mᵤ = aₒ.c² / G = 7.12660796350452E+16 kg see Laws of Motion |
|
Nobility |
Describes the chemical stability of a metal. The more noble a metal (e.g. gold, silver, platinum, etc.) the less chemically active it is. The least noble metals, which include caesium, sodium and magnesium are all highly reactive. A noble metal is electrically positive; i.e. it has a high affinity for electrons (see electronegativity). As a rule, the higher the electronegativity of a metal, the more noble it is. Therefore, whilst electronegativity cannot be used as a measure of nobility it may be used as a guide. |
|
Nodal Points |

Two points on the axis of a Lens such that an entering ray of light would theoretically project through to one nodal point (ignoring refraction) and appear to exit the lens from the other nodal point, parallel to the entering ray. The angle at which a such a light-ray must enter the lens varies with its radial distance from the lens axis and only occurs at radii close to it. The primary nodal point refers to the point at the front of the lens As the radial distance (from the lens axis) of the entering light-ray tends towards zero, the nodal point (primary or secondary) of a lens will coincide with the associated principal point if the refractive index of the environments are identical both sides of the lens. |
|
Node |

The points of intersection at each end of a mode |
|
Non-Polar |
All-pervasive: acts in all directions at the same time Non-polar electro-magnetic values only attract. see also Polar (directional) and Polar (electro-magnetic) |
|
Normal |
'Normal to' is an expression used to describe the relationship of one direction being at right angles to another |
|
Normalise |
To normalise a steel alloy is to optimise its strength by heat treatment without making the material hard (brittle). Carbon steel is normalised by heating it above 723°C, holding it there to allow all the material to transform to austenite and allowing it to cool naturally in still air, i.e. more quickly than annealing. |
|
Nucleus |
The proton partners of all the proton-electron pairs comprising an atom, together with their attached neutrons, which may be one or two per proton-electron pair. The structural arrangement of the atomic nucleus will define the lattice structure of the same atoms in both gaseous and viscous conditions. |
|
Oblique |
An angle that is not equal to 90° |
|
Oblique (triangle) |
A triangle in which none of the angles equal 90° |
|
Oblong |
A three-dimensional, six-sided figure, each side of which is 90° to its adjacent sides see also Rectangle |
|
Obtuse (angle) |
An angle between 90° and 180° |
|
Open Hearth |
The Open Hearth is a process for making steel (it is also called the Siemens-Martin process). It directs the waste heat given off by the furnace through an open firebrick lining, heating the brick-work to a very high temperature. The same pathway is also used for introducing preheated air into the furnace significantly increasing the melt temperature. This process is gradually replacing the Bessemer Converter. |
|
Orbit |
There are three distinct categories of orbit, each of which possesses a unique characteristic: Circular; e = 0 Elliptical; 0 < e < 1 Linear; e = 1 'e' = orbital eccentricity see Laws of Motion |
|
Order |
see Disorder |
|
Organic |
Elements that can intrinsically self-replicate. They generally (but not only) include carbon molecular chains. |
|
Overpressure |
A pressure higher than ambient It is normally measured in multiples of unit ambient pressure such as bar or atmospheres, e.g... |
|
Ozone |
Oxygen molecules (O₂) are split apart by the sun's ultra-violet light radiation and the two resultant oxygen atoms then bond to other oxygen molecules to produce ozone (O₃). Ozone is unstable and converts back to oxygen molecules as it falls into the troposphere where it reacts with other gases. see Ozone Layer for an explanation of the 'hole'. |
|
Pappus theorum |
The calculation of volumes by rotating an area about a central axis E.g. V = 2πRA |
|
Parabola |

The perimeter of any plane cut through a right-circular cone parallel to its slope where; y² = 2.p.x see Elliptical Curves |
|
Parallel |
Two infinitely long 1-D or 2-D shapes that will never converge irrespective of their proximity. |
|
Parallelepiped |
Any three-dimensional figure with six faces, four of which are dimensionally equal and parallel and the two ends are also parallel and dimensionally equal. Also described as a three-dimensional rectangle of constant thickness. A special Parallelepiped is a cube where all 6 faces are dimensionally equal. |
|
Parallel Lay |
All strands are wrapped parallel to each other, i.e. in the same helical direction |
|
Paramagnetism |
The attraction of a non-magnetic material towards a magnet |
|
Paraxial |
A ray of light travelling towards the front face of a lens very close to its axis and parallel to it. |
|
Partial Pressure |
The pressure of each gas occupying a space or container in a mixture of fluids see Partial Pressure-technical help |
|
Particle |
The smallest (and only) components of an atom: see also Quanta |
|
Pauli's Exclusion Principle |
Only one fermion can occupy a quantum state at a given time |
|
Pearlite |
Alternate layers of cementite and ferrite generated as austenite containing 0.83% carbon cools below 723°C Pearlite contains the same amount of carbon as the maximum carbon carrying capacity austenite (0.83%) |
|
Perigee |

The nearest point an orbiting body gets to its force-centre Whilst this term can also be used instead of Perihelion (i.e. it means the same thing), it is normally used to describe the point at which the distance between earth and its orbiting moon is smallest a = half the width of the elipse |
|
Perihelion |

The nearest point an orbiting body gets to its force-centre Whilst this term can also be used instead of Perigee (i.e. it means the same thing), it is normally used to describe the point at which the distance between sun and its orbiting planet is smallest a = half the width of the ellipse |
|
Permeability |
see magnetism and magnetic field |
|
Permeance |
A measure of the ability to transfer magnetic charges (electrons) between adjacent atoms. see magnetism |
|
Permittivity |
The ratio of electric displacement in a medium to electric field intensity producing it. The permittivity of free space: Because the speed of light and the magnetic constant are both exact values, this too is an exact value. |
|
Perpendicular |
At 'a right angle' or '90°' or 'normal' to a line or surface |
|
PERS |
Potential energy recovery system. The formula for potential energy is m.-a.d |
|
Phase Angle (electrical) |

The angle of shift between the alternating current and the voltage in an AC power supply. As the current in an inductive AC power supply will peak after the voltage, it is said that the current 'lags' the voltage. As the current in a capacitive AC power supply will peak before the voltage, it is said that the current 'leads' the voltage. If there is only inductive or capacitive resistance in an AC power supply, i.e. there is zero resistance in the conductor, the phase angle will be 90°. If there is no inductive or capacitive resistance in an AC power supply the phase angle will be 0°. Electrical resistance in the conductor will shift the phase angle between 0° and 90°. The Arccosine of this 'Phase Angle' is the Power Factor of the supply. see also Apparent Power and True Power |
|
Phase Diagram |
An equilibrium diagram of an alloy metal that defines the natural crystal structure of specific alloy compositions at specific temperatures. see Copper Alloys, Metal Properties and Carbon Steels |
|
Photon |
An electron that emits electromagnetic radiation (light) equal to Planck's constant multiplied by its frequency A photon is a Gauge Boson m=0g, Ø≈0m, lifetime<10¹⁸yrs, Q=0, Iz=1 All of which is fiction. Electrons do not emit light (see Quantum Theory is Dead and Relativity is Dead). |
|
Physics |
The prediction of natural characteristics or events using mathematics. |
|
pi (π) |
The ratio between a circle's circumference and its diameter π is an infinitely long number that can be calculated from the series formula: CalQlata uses the value of 3.14159265358979 in all its calculators. |
|
Pinned Support |
A simple support on a beam |
|
Pipe |
A tube or cylinder with a length greater than ten times its diameter. see Thin-Wall Tube |
|
Pitch (vessels) |

The rotational movement of a vessel about the lateral ('y') axis Whilst this term generally applies to the movement of ships, it is also used to define the movement of any object subject to the same degrees of freedom; e.g. aircraft and spacecraft see Right-Hand Rule for positive and negative directions of this movement |
|
Planck's Constant |
Planck's law states that the energy of an electromagnetic wave is confined to indivisible packets (quanta) each of which has to be radiated or absorbed as a whole and the magnitude of this energy is proportional to its frequency. If E is the energy (J) and ƒ is the frequency of the electromagnetic radiation (/s) then: However, the units achieved by his formula and those he claimed for it; 'J.s', are incompatible. Planck's constant is incorrect. The modified (corrected) version of Planck's constant applies to electro-magnetic energy see also Planck's Atom |
|
Planck's Atom |
Planck's atom comprises three values he defined; time, length and mass, each of which is calculated as follows: Planck's atomic particle: see Planck's Atom |
|
Plastic Strain |
Matter that does not return to its pre-deformation dimensions when stress is released |
|
Plow Steel |
A high quality, high strength carbon steel (0.5% to 0.95%) used to manufacture 'Bead Wire' |
|
Point Load |
An applied force that is concentrated at a single point |
|
Poisson's Ratio |
The relationship between axial stress and the lateral deformation when applied to a body that obeys Hooke's Law; Poisson's ratio varies in magnitude between 0 for a totally brittle body (no deformation whatsoever) and 0.5 for a perfect elastomer (same deformation in all three directions). 0.3 is normally used for carbon steel |
|
Polar |
Unidirectional: acts in a straight line see also Non-Polar |
|
Polar |
Positive or negative value Like polar values repel, and unlike polar values attract. see also Non-Polar |
|
Polar |

The directional definition of a vector using its angular relationship to 2-D (α,r) or 3-D (α,β,r) axes see also Cartesian Co-Ordinates and Vector |
|
Polar Moment of Inertia |
The structural resistance of a cross-sectional area to torsional deformation The polar moment of inertia of any shape is the sum of any two second moments of area (of the same shape) at 90° to each other |
|
Polygon |
A 2-D shape with three or more sides A Regular polygon has equal sides and an irregular polygon has unequal sides |
|
Polytropic |
A process in which the relationship between pressure, volume and ratio of specific heats of an ideal gas in a system remain constant and can be defined thus: p.Vn = K Where: see Thermodynamics |
|
Positron |
A positive electron of the same mass and electrical charge (but opposite sign) as an electron Positrons are produced in the decay of radio-isotopes and X-rays of energy greater than 1MeV |
|
Potential-Difference |
The potential energy per electron (Joules per Coulomb) across two discrete points in an electrical circuit; also referred to as Voltage (Volts) and electro-motive force. The unit of measurement is a Volt which equals one Joule of work done by one Coulomb of electrical charge. |
|
Potential Energy |
The energy stored in a system or body It could be stored in a compressed (or stretched) spring: Where: |
|
Potential-Hydrogen |
Potential Hydrogen (sometimes referred to as the 'power of hydrogen') defines the positive hydrogen ion activity in a solution pH can be calculated using the following formula: |
|
Power |
The rate at which energy (or Work) is expended. The faster work is done, the more power is consumed For eaxmple, the power of; The units of measurement are; Btu/h, W, J/s, cal/min, N.m/s, lbf.ft/min etc. |
|
Power |
The reciprocal of the focal length (ƒ) of a lens |
|
Power Factor (electrical) |
The factor applied to the Apparent Power of an alternating circuit in order to convert it into True Power It is calculated thus: p.f. = Resistance ÷ Impedance and is the cosine of the Phase Angle between the alternating current and the alternating voltage. |
|
Pressure |
A force applied over an area. |
|
Pressure Vessel |
An enclosure of any shape that will contain or resist a fluid under pressure without leakage Thin-wall vessel: t < Ø÷10 |
|
Pressure Welding |
Press two or more metals together with sufficient pressure to cause metal fusion between them. |
|
Primary Stress |
The three uni-directional tensile stresses oriented along the 3-D axes (see Right-Hand Rule) of a mechanical component |
|
Principal Planes |

The curved planes that trace the principal points of light-rays entering a lens parallel to its axis. The primary principal plane refers to the plane at the front of the lens |
|
Principal Points |

The theoretical points of intersection of light-ray projections after entering and before exiting a lens. The primary principal points refers to those at the front of the lens see also Principal Planes and Nodal Points |
|
Principal Quantum Number |
The first in a set of numbers that uniquely describes the quantum state of an electron The principal quantum number of an electron can only be a positive integer and identifies the shell number it occupies: All other electrons in the same shell will have the same principal quantum number but at least one of its other quantum numbers must be different |
|
Principal Stress |
A primary stress reoriented (in space) and altered in magnitude as a result of simultaneous shear stresses |
|
Prism |
A longitudinal body with sides of equal width and constant cross-section. The term for a prism usually ends with '....hedron'. For example: |
|
Process |
Any alteration in the energy of a system Any change in the physical properties of a system will require a change in its energy, either by addition or removal Reversible Process: Irreversible Process: |
|
Prograde |
The same direction of rotation as the spin (rotation) of a force centre For example, the sun in our solar system spins in a clockwise direction looking down on top of it see also Sidereal and Retrograde |
|
Proton |
A single indivisible packet of electro-magnetic charge that possess no intrinsic kinetic energy. mass; mₚ = 1.67262163783E-27 kg see The Proton-Electron Pair |
|
Proton Charge |
A proton possesses both magnetic charge and electrical charge. Magnetic charge is today referred to as mass. In a proton, magnetic charge is constant irrespective of temperature; Electrical charge in a lone proton is numerically equal and opposite (positive) to the elementary charge unit. The maximum eꞌ value (at the neutronic temperature) is 'eₙ' see The Proton-Electron Pair and Mass is Magnetic Charge |
|
Proton-Electron Pair |
A proton with an orbiting electron. see The Proton-Electron Pair |
|
PVRT |
Reference to an established calculation method that was originally created for the properties of a gas, The ideal gas law was created in 1834 by Benoît Paul Émile Clapeyron from the work done by; see also Thermodynamics and State of Matter |
|
Pyramid |
A regular or irregular three-dimensional prism or cone with a pointed cap see also Frustum |
|
Quadrangle |
An irregularly shaped four sided figure |
|
Quadrilateral |
A regularly shaped four sided figure CalQlata consider all quadrilaterals to be four-sided figures with all internal angles equal to 90° |
|
Quanta |
A collection of more than one particle |
|
Quantum Number |
One of a set of numbers that uniquely describe the quantum state of an electron |
|
Quark |
The family of sub-atomic particles within the fermion group that constitute the constituents of matter Quarks combine to form composite particles called hadrons, the most well-known of which are protons and neutrons |
|
Quench |
The rapid cooling of a heated steel alloy to lock in an unnatural crystal structure to harden and strengthen it. |
|
Radial |
At right-angles to a central axis |
|
Radial Stress |
Primary stress at right-angles to (out-of-plane with) both longitudinal and lateral (or hoop) stresses |
|
Radian |
An angle expressed in terms of π π radians = 180° |
|
Radiation |
The emittance of electro-magnetic energy from a proton-electron pair. 1 Roentgen corresponds to the production of CalQlata has discovered a radiation constant that it has yet to explain: |
|
Radical |
A number multiplied by itself (as many times as you wish) |
|
Radicand |
A number within a root |
|
Radioactivity |
The splitting of a neutron into its component parts; a proton-electron pair. The energy released will either be as; The EME will be released as heat, and the atom's atomic number will increase by one for each neutron split, |
|
Radio Waves |
Very long-wave electromagnetic radiation used for the transmission of sound λ > 0.1m (ʄ < 2.99792459E+08Hz) |
|
Radius of Gyration |
The distance from an axis of rotation to the centre of area (or mass) ɍ = √(I/A) and ɍ = √(I/m) |
|
Ratio of Specific Heats |
γ = cp ÷ cv pure monatomic gases: γ ≈ 1.67 see Heat |
|
Reactance (electrical) |
Reactance ('X') of inductance ('L') or capacitance ('C') is their resistance equivalent in an AC current. where: ƒ = frequency |
|
Recrystallisation |
The movement of atoms in a metal lattice structure towards a low energy position. After cold working or heat treatment, a residual stress is generated in a metal by atoms having been forced away from their natural position within the lattice structure. |
|
Rectangle |
A two-dimensional, four-sided figure, each side of which is 90° to its adjacent sides see also Oblong |
|
Refractive Index |
The ratio of the phase velocity of electromagnetic radiation in a vacuum and its phase velocity in a medium For example: see also Snell's Law |
|
Refraction |
The bending of light (or any electromagnetic wave) by altering its velocity; e,g, as it passes between media of different densities. see also Refractive Index |
|
Regular Lay |
A particular type of helical lay pattern in a wire rope where the filaments are wrapped within each strand in the opposite helical direction as the strands are wrapped within the wire rope. In a manufactured wire rope, the filaments run parallel with the long axis of the wire rope (see image). 
You can have 'right lay' or 'left lay' configurations: Whilst these wire ropes provide less wear resistance than 'Lang lay' constructions due to their undulating (peaky) surface and lower bending fatigue resistance, the structure is much more stable and resistant to abuse. Regular lay wire ropes are generally used for all non-specialist applications. see also Lang Lay |
|
Relative Atomic Charge |
The mol-charge capacity of sub-atomic particles: Charge rate: q = RAC/RAM {C/ It is currently held that the proton carries the same charge as an electron as shown above, but CalQlata disagrees. |
|
Relative Atomic Mass |
The mass number of the most stable isotope of an element For example, if Iron (Fe), which has an atomic number of 26, had the same number of protons and neutrons, its RAM would be 52 yet its actual value is 55.847. This means that in its most stable condition Iron's atoms contain more neutrons that protons and that this excess varies in any bulk of its material. electron: RAMₑ = 0.000548580318390698 g/mol |
|
Relative Charge (capacity) |
The electrical charge capacity of matter: The reciprocal of this property and value is today referred to as Lorentz's magnetic field; B = 1/RC. see also Magnetic Charge |
|
Reluctance |
A measure of the resistance to the tansfer of magnetic charges between adjacent atoms. see magnetism |
|
Resistance |
The reciprocal of Conductance The pipeline analogy for resistance is an internal restriction such as a helical vane or venturi both of which restrict flow but provide work and generates the pressure drop across the inlet and outlet of the pipe. R = 1/G = V/I {J.s / C²} Electrical resistance is directly proportional to the length of a conductor, and inversely proportional to its cross-sectional area. |
|
Resistivity |
The reciprocal of Conductivity |
|
Resistor |
A material that prevents movement of its electrons when exposed to a potential difference This term is relative (see Conductor). You can have a good resistor and a bad resistor. The better the resistor, the harder it is for its electrons to flow |
|
Resonance |
A vibration that occurs in a sprung system at or close to its natural frequency during which deflections are amplified |
|
Response Amplitude |
The amount of movement in a vessel at sea (linear and rotary) generated by a passing hydrodynamic wave in six degrees of freedom |
|
Response Amplitude Operator |
A factor that must be multiplied by a wave height or slope (wave or water) in order to define a response amplitude Also called a 'transfer function', which refers to the transfer of motion in a specified wave to the motion of the body (e.g. ship) |
|
Rest Mass |
The mass of a stationary body |
|
Retrograde |
The opposite direction of rotation as the spin (rotation) of a force centre For example, the sun in our solar system spins in a clockwise direction looking down on top of it |
|
Rhombic |

Describes the lattice arrangement of atoms within a metal. The smallest crystal of this metal will contain eight closely packed atoms, each of which are located at each corner of a cube that has slipped to form a rhomboid prism. |
|
Right-Angle |
An angle of exactly 90° |
|
Right-Hand Rule |

The thumb, index and middle fingers of the right hand are projected at 90° to each other in three dimensions, where the index finger represents the x-axis (Surge), the middle finger represents the y-axis (Sway) and the thumb represents the z-axis (Heave) 
This definition also refers to the direction of rotational twist about the vector axes; the thumb of the right hand is projected in the direction of the axes defined above and the four fingers naturally curled in towards the palm of the hand. The positive direction of rotation {xy(Yaw), xz(Pitch), yz(Roll} are in the direction of the four fingers |
|
Right-Angle Triangle |
A triangle with one of its angles at exactly 90° |
|
Roentgen [Röntgen] |
The unit of radiation exposure, dedicated to the discoverer of x-rays (Wilhelm C Röntgen). Also described as the charge per unit mass (of air), equal to 2.58E-04 C/kg, generated by ionisation from x or γ-radiation |
|
Roll (vessels) |

The rotational movement of a vessel about the longitudinal ('x') axis Whilst this term generally applies to the movement of ships, it is also used to define the movement of any object subject to the same degrees of freedom; e.g. aircraft and spacecraft see Right-Hand Rule for positive and negative directions of this movement |
|
Rotational Acceleration |
The equivalent linear acceleration of a point orbiting its force-centre (i.e. assuming the orbital path to be straightened out) see also Linear Acceleration and Angular Acceleration |
|
Rotational Energy |
rotational kinetic energy; or the energy required to spin a body ay velocity; 'ω' U = ½.I̊.|ω|.ω |
|
Rotational Velocity |
see tangential velocity and angular velocity |
|
Rotor |
The rotating part of an armature in a motor, alternator or generator. see also stator |
|
Rupture Modulus |
The limiting tensile stress in wood under bending. |
|
Rydberg Formula |
Johannes Rydberg developed his formula to calculate the wavelength of a photon that would be emitted when an electron drops from a high initial energy state (shell nᵒ: n₁) to a low final energy state (shell nᵒ: n₂), which can be calculated thus: Rydberg's universal constants for the limiting conditions are provided below: Rydberg's orbital constant was discovered only recently, but it was derived from his original work. Whilst it applies primarily to his orbital radius (aₒ), it also appears in other constants including the coupling ratio; Maximum wave number: Electron energy at ground state: Nils Bohr developed alternative values for R∞ and Rᵧ based upon Rydberg radius (orbital): |
|
Safety Factor |
The proportion of additional stress (or use) that can be applied to a material (or component) before it fails. For example: if you design a component to experience less than or equal to 200 N/m² of stress and the material from which it has been manufactured has a minimum specified yield stress of 400 N/m², the component has a safety factor of 2 Safety factor is the inverse of utilisation |
|
Satellite |
A mass orbiting a force centre under the influence of gravitational force. For example: |
|
Saturation |
The ability of a fluid to hold another substance (gas, liquid or solid) in suspension that varies with pressure and temperature High temperatures low pressures raise the saturation level |
|
Scalar |
A quantity (magnitude) that has no direction, such as; see also Vector |
|
Schwarzschild |
Karl Schwarzschild's formula to determine the radius of a mass that would prevent the escape of photons from its surface: Concerning the outer radius (R) of any given mass; Schwarzschild derived his formula from Newton's formula for escape velocity: Schwarzschild's radius for any mass of ultimate density: Whilst his formula is correct, it applies to only to the fictitious photon. |
|
Science |
A generic term that encompasses the study of nature. |
|
Seafastening |
Welds, padeyes, lugs and other deck attachment facilities used to fix a mass to any part of a ship or vessel in order to prevent unwanted movement whilst in transit at sea |
|
Seastate |
The properties of a sequence of following waves defining representative conditions used for equipment design and/or operational conditions. Seastates are usually compiled from actual data collected from anchored 'buoys' installed in various regions around the world and subsequently sorted seasonally, monthly or daily. Representative seastates are used for operational, design (e.g. fatigue calculations), extreme, etc. wave sequences for specified weather windows. |
|
Second Moment of Area |
Describes the structural strength of a cross-sectional area, or a numerical representation of its resistance to bending deformation see also Moment of Inertia and Polar Moment of Inertia |
|
Section Modulus |
Describes the ratio of the second moment of area to the distance from the neutral axis of the stressed plane (I/y) |
|
Sector |

A shape enclosed by two radii and an arc of the same circle |
|
Segment |

|
|
Self-Holding |
When referring to Tapers (Morse, Jarno, Brown & Sharpe, etc.), "Self-holding" describes a condition whereby the assembly (insertion) force is the same as the disassembly (extraction) force and the coefficient of friction between tapered shaft and mating sleeve materials holds them together during use. |
|
Shatter |
Break apart (usually into small pieces) without deformation. |
|
Shear Angle |
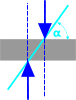
The angle between two parallel planes and two opposing forces. see shear force, shear stress and shear modulus. |
|
Shear Force |
Equal and opposite tangential forces induced in a substance between two parallel planes. |
|
Shear Modulus (G) |
see Modulus of Rigidity. |
|
Shear Stress |

Stress induced in a substance due to a shear force and a shear angle. τ = F / A.Sin(α) |
|
Sidreal |
The actual rotation of a body, e.g. a planet, with respect to a fixed point in space This is not the same as the rotation of an orbiting body with respect to its force centre see also Prograde and Retrograde |
|
Simple Support |
A support that prevents only one degree of freedom, which is at right-angles to the load |
|
Simpson's Rule |
An algebraic solution for integrals that are difficult or impossible to solve by integration |
|
Single-Phase |
The flow of a single category of fluid. Normally applied to the flow of fluids through a pipe in the petroleum industry; see also Multi-Phase |
|
Sinter |
The coalescing (conglomeration) of solid particles using heat but without actually melting (liquifying) the material. |
|
Sinusoidal |
A continuous curve with the same x,y relationship as the Sine of an angle |
|
Slag |
The unwanted (remnant) material left over after extracting a material from an ore. Slag contains commercially valueless material and an amalgamation flux. |
|
Slugging |
The thickening or solidification of a heavy liquid in a pipe or constricted passage that prevents free-flow conditions. |
|
Smelt |
The extraction of a metal from an ore by melting. During melting, the ore will separate into two layers: a lower layer of molten metal and an upper layer of slag. |
|
S-N Curve |
The graphical representation (normally a straight-line Log10-Log10 plot) of maximum allowable stress range plotted against the maximum possible number of cycles (for this stress range), above which, damage to the material or mechanical component is expected (S-N = stress number, i.e. the number of stress cycles) |
|
Snell's Law |

Willebrord Snellius: Dutch Professor of mathematics at the University of Leiden Snell's law (1621) states that after passing from one medium of refractive index 'n₁' into a medium of refractive index 'n₂' a ray of light will refract according to the following relationship: |
|
Solid |
Matter in a viscous state such that inter-atomic strength is sufficient to resist gravitational forces. # see State of Matter |
|
Solubility |
The ability of one substance (the solute) to dissolve onto another substance (the solvent) This ability may be measured in terms of moles per cube (e.g. moles/cm³) |
|
Sonic |
Refers to a condition that generates a sound. Whilst this velocity varies with altitude (depth), the speed of sound is generally quoted as; # see Speed of Sound |
|
Sound |
Refer to Sonic. |
|
Spar |
A principal structural member of an aircraft wing or aerofoil |
|
Special Relativity |
A theory based upon the deformation of time that redefines the speed of objects moving close to the speed of light as: see Relativity is Dead |
|
Specific Charge(s) |
|
|
Specific Charge Capacity |
The quantity of electrical charge energy per unit temperature, per unit mass, that can be absorbed by a specific substance. Specific charge capacity of atomic particles: The most common units of measurement are J / C.K, ft-lb / C.°R, Btu / C.°R, etc. see also Charge Capacity, Specific Heat Capacity and Specific Heat Ratio |
|
Specific Enthalpy |
The enthalpy per unit mass of a system h = u + Rₐ.Ṯ The units of measurement are J/g, Btu/lb, etc. see Heat |
|
Specific Entropy |
The entropy per unit mass of a system s = KB.ln(N) = cp.ln(Ṯ) {per molecule} The units of measurement are J/K/mol or J/K/g, Btu/°R/lb-mol or Btu/°R/lb, etc. see Heat and Thermodynamics |
|
Specific Gravity |
Also called 'Relative density', is the density of a liquid or a solid relative to pure water (1000 kg/m³). For example, the SG of steel is 7.85 (7850 kg/m³ divided by 1000 kg/m³) The specific gravity of a gas is quoted relative to the density of air (1.297 kg/m³). For example, the SG of helium is 0.1365 (0.177 kg/m³ ÷ 1.297 kg/m³) |
|
Specific Heat(s) |
|
|
Specific Heat Capacity |
The quantity of heat energy per unit temperature, per unit mass that can be absorbed by a specific substance. Specific heat capacity of atomic particles: Specific heat capacity of dry air: The most common units of measurement are J/kg/K, W.s/kg/K, BTu/lb/R, etc. see also Heat Capacity, Specific Charge Capacity, Specific Latent Heat and Specific Heat Ratio |
|
Specific Heat Ratio |
The ratio of specific heat capacities: γ = cᵨ:cᵥ see State of Matter |
|
Specific Internal Energy |
The internal energy per unit mass of a system u = Ṯ.cᵥ The units of measurement are J/kg, Btu/lb, etc. see Thermodynamics |
|
Specific Latent Heat |
The quantity of heat energy per unit temperature, per unit mass that changes the state of matter. The most common units of measurement are J/kg, W.s/kg, BTu/lb, etc. see also Heat Capacity, Specific Heat Capacity and Specific Heat Ratio |
|
Specific Volume |
Volume per unit mass, or... |
|
Specified Minimum Yield Stress |
The minimum yield strength (maximum allowable yield stress) for a material as specified by a recognised authority |
|
Spheroidising |
The balling up of the cementite in pearlite during the annealing process |
|
Spin Energy |
The energy generated by a rotating body of a specific polar moment of inertia: E = ½.J.ω² |
|
Spin Quantum Number |
The fourth in a set of quantum numbers that describes the spin direction of the electron The spin quantum number of an electron can be a positive or negative half integer: i.e. -½ or +½ The spin quantum number must be different for each electron in the same shell with the same azimuthal and magnetic quantum numbers |
|
Spring Coefficient |
The proportional relationship between force and movement for an elastic material or a spring Also referred to as a spring constant and spring rate Measured in e.g. N/m (lbf/in) see also Stiffness |
|
Spring Constant |
|
|
Sprung System |
Any machine or structure, or part thereof, that obeys Hooke's Law, which includes most metals, elastomers (rubber), ceramics, hard-woods, etc. |
|
Spur Gear |
A gear wheel, with teeth cut into its circumference at 90° to the plane of the wheel, that normally (but not necessarily) mates with another spur gear Also referred to as straight-cut gear The centres of rotation of two mating spur gear wheels are always parallel |
|
Stanchion |
A vertical structural member |
|
Station-Keeping |
A satellite's ability to maintain its orbital path; see Laws of Motion |
|
Stator |
The stationary part of an armature in a motor, alternator or generator. see also rotor |
|
Steady Flow |
A fluid flowing through a system the conditions and properties of which do not change with time. |
|
Stiction |
Stiction (static friction) is the resistance to initiate relative movement between two surfaces when forced together Stiction is always greater than friction |
|
Stiffness |
The resistance any material or structure exhibits to deformation Stiffness can be applied to bending, torsional and axial deformation and describes the rate of dimensional change for a specified force see also Spring Coefficient |
|
Strain |
The elongation in a material as a result of stress see also Elastic Strain and Plastic Strain |
|
Strand |
More than one filament wound together see also Wire Rope |
|
Strange Quark |
The third lightest of all the quarks and part of the second generation of matter The strange quark is classified as a fermion mᵣ=1.693528659E−25g [95.0MeV/c²], Q=-⅓e, Iz=-½ |
|
Stratosphere |
The ceiling for 99% of nitrogen, the earth's most abundant atmospheric gas Altitude @ equator: 15km to 60km Stratum of the earth's atmosphere that comprises mainly nitrogen and oxygen and is considered to be the upper limit of the atmosphere that contains anything of substance Temperature increases with increasing altitude |
|
Stress |
Force per unit area [in a substance]. the general formula for this property is; Tensile stress: induced in a material as a result of a positive axial load Failure in materials due to elastic stress is predicted using various theories: Principal stress (Rankine), Principal strain (e.g. St Venant) or strain energy (Beltrami and Haigh), shear (e.g. Tresca) or shear strain energy (Henckey & Von Mises) |
|
Stress Amplitude (Sa) |
Half the stress range (Sa = δS÷2) |
|
Stress Block |
A collection of characteristic identical stress ranges that represent a defined period of time in the fatigue life of a material or component |
|
Stress Corrosion Cracking |
The cracking or 'crazing' of a metal surface due to galvanic corrosion whereby the cracks generate stress-concentrations in the material weaking its ability to deform elastically Hydrogen emrittlement is a typical example of this problem (see Cathodic Protection) NACE MR0175/ISO 151546-1:2001(E) are internationally recognised standards that offer advice as to the control of this problem |
|
Stress Cycle |
One stress cycle is twice the stress range (e.g. maximum to minimum and back to maximum again) |
|
Stress (mean) |
Middle of the the stress range (Sm = (Ṡ+Ṣ)÷2) |
|
Stress Range |
The difference between the maximum stress and the minimum stress in a single stress cycle (δS = Ṡ-Ṣ) |
|
Subduction |
The passing of planet's crust under a tectonic plate and down into its mantle Regions where this occurs are called Subduction Zones, and they are usually active with volcanoes and earthquakes |
|
Sublime |
The transition of matter between viscous and gaseous states without melting. |
|
Subsonic |
Refers to a velocity less than Sonic. see also Hypersonic, Supersonic, Transonic |
|
Substance |
The bulk quantity of particles in a viscous state. see also Matter |
|
(the) Sun |
including atomosphere (excluding atmosphere) Principal constituent elements (99.998%): |
|
Supersonic |
Refers to a velocity greater than Sonic. see also Hypersonic, Subsonic, Transonic |
|
Surface Energy |
The free energy per unit area in a substance US = surface tension x surface area |
|
Surface Tension |
A property of liquids whereby their surface appears to have an elastic membrane in a state of tension This phenomenon occurs at the surface of liquids due to unbalanced cohesive forces between surface molecules that do not occur in the bulk of the liquid where molecular cohesive forces are able to act in all directions. |
|
Surge (vessels) |

The linear movement of a vessel in the direction of the longitudinal ('x') axis Whilst this term generally applies to the movement of ships, it is also used to define the movement of any object subject to the same degrees of freedom; e.g. aircraft and spacecraft see Right-Hand Rule for positive and negative directions of this movement |
|
Sway (vessels) |

The linear movement of a vessel in the direction of the lateral ('y') axis Whilst this term generally applies to the movement of ships, it is also used to define the movement of any object subject to the same degrees of freedom; e.g. aircraft and spacecraft see Right-Hand Rule for positive and negative directions of this movement |
|
Sweating |
Line (or coat) the surfaces of two metals with melted solder or braze and allow to cool. |
|
System |
Thermodynamic: A quantity of matter subjected to a process, everything else is its surroundings Mechanical: The components that together create a particular action Chemical: The molecules, atoms, sub-atomic particles and electro-magnetic radiation that are responsible for a chemical reaction see Thermodynamics and Sub-atomic Physics |
|
Symmetry |
The matching of opposite sides or faces. Symmetrical systems are achiral systems. |
|
Tangential Velocity |

The instantaneous linear velocity of an orbital particle or satellite, which may be calculated as follows: see also Angular Velocity, Linear Velocity and Rotational Velocity |
|
Tauon |
A negatively charged elementary particle also referred to as a tau particle The tauon and its anti-particle carry the same electric charges as the electron and positron respectively A tauon is classified as a lepton m=3.167469045E-24g [1.77682GeV/c²], lifetime≈2.9E-13s |
|
Tectonic Plate |
An area of planet crust that changes size and shape over time due to mantle activity Normally, a length of the tectonic plate's perimeter (edge) grows through new rock generation from volcanic emissions and the remaining perimeter is subject to subduction |
|
Telescope |
A means of increasing the visibility of distant objects. Optical Telescope: a means of detecting distant objects within the visible spectrum using optical lenses. Radio Telescope: a means of detecting the EME of distant objects outside the visible spectrum. |
|
Temper |
Tempering is carried out to increase toughness and ductility in a material that has been previously hardened. The tempering process is carried out by heating the material to a temperature below its transformation temperature (1330°F or 723°C) and cooling in a controlled environment/manner to allow some recrystallisation to occur. |
|
Temperature |
The heat energy in electro-magnetic energy. The degree of heat measured relative a particular scale The two most commonly used 'absolute' scales are: The two most commonly used 'non-absolute' scales are: To convert between scales: 0K = 0°R = -273.15°C = -459.67°F Key Temperatures: see The True Atom |
|
Temperature Constant |
Temperature constant used to define electrical force of repulsion between adjacent atomic nuclei: |
|
Tenacity (t) |
The tensile strength of a single fabric thread in grams force per denier (gfpd). Note the average tenacity of: see Fabrics see also Denier |
|
Tensile Modulus |
A general term that applies collectively to the resistance to axial deformation for all materials (e.g. metals, polymers, woods, etc.) including; Young's, flexural and elastic moduli. |
|
Tesla |
The conventional unit of measurement for what is described as 'magnetic field strength' (B); kg/C/s. see Magnetism. |
|
Tetra-hedra |

Describes the lattice arrangement of atoms within a metal. The smallest crystal of this metal will contain four closely packed atoms, each of which are located at each corner of a tetrahedron. |
|
Thermal Conductivity |
The rate at which heat flows through a material or substance of unit thickness. |
|
Thermal Expansion |
The rate at which matter changes in size per degree of change in temperature; e.g. /°C, /K /°F, /°R, etc. The coefficient is specified in units of length; e.g. γ for steel is 1.17E-05 /°C (6.5E-6 /°F) |
|
Thermal Resistance |
The temperature difference across a barrier of a specified thickness when a unit of heat flows through it in unit time. |
|
Thermodynamics |
The theory of the transfer of energy between systems or its conversion into work In other words ... see Thermodynamics |
|
Thermosphere |
Stratum of the earth's atmosphere that contains the Ionosphere (Lower Thermosphere) and the Exosphere (Upper Thermosphere) Thermosphere means 'hot region'. The temperature of this region increases exponentially with altitude but the density of material in this stratum is so thin that the heat generated cannot be readily transferred. |
|
Thin-Wall Tube |
A pipe or cylinder, the nominal radius (r) of which is greater than ten times its wall thickness (t). In reality, this definition varies throughout industry, dependent upon the reference documentation, some of which are listed below: |
|
Through Harden |
A process whereby the entire body of a steel alloy is hardened to the same extent and is normally achieved by the addition of alloying elements. |
|
Top Quark |
The most massive of all the quarks and part of the third generation of matter The top quark is classified as a fermion mᵣ=3.085252685E−22g [173.07GeV/c²], lifetime≈5E−25s, Q=⅔e, Iz=½ |
|
Torque |
A rotational moment This is a tangential force applied to a structural element at a given radial distance from the axis of twist. |
|
Torsion |
The act of applying a torque to induce stress or tighten a screw. |
|
Transfer Function |
|
|
Transformation Temperature |
The temperature range during which austenite occurs during heating. It is also a different temperature range during which Austenite disappears during cooling. These temperature ranges may overlap but they are never the same. |
|
Transformer (electrical) |
A transformer is a single central magnet around which are wound two or more separate copper wire coils with a different number of turns. The voltage in each coil will vary thus: V2 / V1 = n2 / n1 |
|
Transom |
An horizontal (or transverse) structural member |
|
Transonic |
Refers to a condition in which Subsonic and Supersonic flows are present. see also Hypersonic, Sonic, |
|
Triangle |
A two-dimensional, three-straight-sided closed shape the 3 angles of which add up to 180° (πᶜ) |
|
Triatomic (gas) |
A gas (mixture or pure) comprising only molecules three atoms, such as ozone, water vapour, carbon dioxide, nitrous oxide, etc. |
|
Triple-Point of Water |
273.16K, 0.01°C, 32.018°F & 491.688°R The temperature at which pure water can exist in all of its phases (solid, liquid and gas) simultaneously at 1 atmosphere (pressure) |
|
Tritium (atom) |
A proton-electron pair with two neutrons attached. |
|
Trochoid (curve) |

A trochoid is a curve generated by a point inside or outside the circumference of a circle that is rolled along a flat, straight surface. see also Cycloid, Epicycloid and Hypocycloid |
|
Troposphere |
The ceiling for 75% of nitrogen, the earth's most abundant atmospheric gas Altitude @ equator: 0km to 15km Weather generating stratum of the earth's atmosphere that contains all of the earth's gases Temperature reduces with increasing altitude |
|
True Power (electrical) |
True power is the apparent power multiplied by the power factor and is normally expressed in terms of 'W' or 'kW'. see also Alternating Current and Phase Angle |
|
Turbulent Flow |
A flowing fluid that disturbs and mixes adjacent layers |
|
Ultimate-Body |
All the matter in the universe, comprising more than 2.80059013353655E+75 proton-electron pairs (>4.68687882273808E+48 kg of mass), combined through magnetism that was collectively responsible for the last The Big Bang and includes the body mass of the Great Attractor. see The Universe |
|
Ultimate Tensile Stress |
The maximum stress a material will support before it begins to suffer internal fracturing Resistance to deformation (strength) is increased between yield stress and ultimate tensile stress through work hardening The gradual reduction in strength after reaching UTS is due to increasing internal fracturing which will continue to reduce until the material breaks |
|
Uncertainty Principle |
Any pair of dimensional variables (e.g. time and energy) describing a sub-atomic particle cannot be determined to an accuracy whereby the product (multiplication) of the errors (of each variable) is less than Dirac's constant E.g.: the more precisely the position of a particle is determined the less precisely its momentum can be known, and vice-versa |
|
Universal Period |
The period of time between successive Big-Bangs. see The Universe |
|
Upheaval Buckling (pipeline) |
Lateral displacement of a pipeline due to uncontrolled longitudinal compression. |
|
Up Quark |
One of the two types of quark that make the proton and the neutron and forms part of the first generation of matter The up quark is classified as a fermion mᵣ=3.565323494E-27g [2.01MeV/c²], Q=⅔e, Iz=½ |
|
Utilisation |
The proportion of limiting stress (or use) you design into a component before its material fails. For example: if you design a component to experience a maximum design stress of 200 N/m² and the material from which it has been manufactured has a minimum specified yield stress of 400 N/m², the component has a utilisation of 0.5 Utilisation is the inverse of safety factor |
|
Vacuum |
A volumetric container that contains gaseous matter at below ambient pressure. A perfect vacuum is the same thing but which contains no atomic particles at all, which is impossible to achieve at the surface of a planet. A practical bench vacuum at the surface of the earth is ≈1E-5 bar. |
|
Valence Shell |
The outermost shell of an atom in which an electron exists and includes all sub-shells The valence shell represents an atom's bonding capacity and hence defines its chemical properties. |
|
Vapour |
A liquid in a gaseous state that exists below its critical temperature. In reality, a vapour is a viscous collection of atomic or molecular globules, i.e. a liquid, suspended in a gas. It doesn't become a gas until it exceeds its critical temperature. |
|
Vector |
A straight line action (e.g. force) that has both magnitude and direction see also Polar Co-Ordinates and Cartesian Co-Ordinates |
|
Velocity |
Rate of change in distance. This value may be interpreted as the first derivative of the distance between two points in a journey, which may described mathematically thus: where: see also Linear Velocity, Angular Velocity and Rotational Velocity |
|
Vertex (Vertices) |
The point at which one end of each of two lines connect |
|
Vibration |
The elastic response of a sprung system to repeated loads of similar magnitude and frequency |
|
Virtual Mass |
A shape coefficient that represents the sum of the fluid displaced by the body and the added mass of the surrounding fluid dispersed by the body and its displaced mass |
|
Viscosity |
Viscosity is the measurement of a solid or liquid substance's resistance to shear, and therefore to flow. Kinematic viscosity (ν) is measured in units of m²/s, although it is most commonly measured in Stokes (St) or centiStokes (cSt) where 1m²/s = 1000St Dynamic (or absolute) viscosity (μ) is the kinematic viscosity multiplied by the mass density (ρ) of the fluid and has the units of kg/s/m (or N.s/m²), although it is more commonly measured in Poise (P) or centipoise (cP) where 1N.s/m² = 10P The mathematical relationship is as follows: μ = ν.ρ For example; see The True Atom |
|
Voltage |
Also known as potential difference (pd) and electro-motive force (emf), actually refers to the applied potential energy per electron in a conductor. The pipeline analogy for voltage is the pressure drop across the inlet and outlet of a pipe with a fluid flowing through it. |
|
Vortex |
The rotary motion of a fluid, which may be free or forced. see Vortices |
|
Vortex Shedding |

The generation of 'vortices' (rotating fluid) in the wake of a fluid passing over an object |
|
Vorticity |

A measure of the local angular [rotary] motion of a fluid in a vortex. Anti-clockwise rotation is generally referred to as positive and clockwise rotation as negative. ζ = 2.ω is applicable to rotational vortices (see image) |
|
W Boson |
A weak force carrier There are two types of W Boson Wˉ is the anti-particle of W⁺ m=1.4332244E−22g [80.398GeV/c²], lifetime≈3E-25s, Q=±1e, Iz=1 |
|
Wave Celerity |
The speed (or velocity) of a wave. |
|
Wave Frequency |
The reciprocal of the wave period. |
|
Wavelength |
The speed of a wave divided by its frequency |
|
Wave Number |
The reciprocal of wavelength |
|
Wave Period |
The time taken for a wave to travel through a full wavelength. |
|
Weak Force |
An interaction between elementary particles that is responsible for certain decay processes (such as beta radiation) and acts upon all known fermions Particles interact through the weak force by exchanging W & Z Bosons, which are heavy (≈100 times that of a proton). Their mass defines the short-range (≈1E-17m or ≈100 times less than the diameter of a typical atomic nucleus) nature of the weak force and that makes the weak force appear weak at the low energies associated with radioactivity. |
|
Weather Window |
A period of time during which limiting weather conditions will not be exceeded i.e.; A large weather window means a long period of time over which these limiting conditions are not expected to be exceeded conversely; A small weather window means a short period of time over which these limiting conditions are not expected to be exceeded |
|
Weber |
The unit of measurement for magnetic flux. |
|
Weight |
The resultant force between two bodies as a result of gravitational acceleration and is measured in N, pdl, etc. The weight of a body is equal to its mass multiplied by the gravitational acceleration. This property varies with gravitational acceleration. That is, any given mass will weigh differently on the Earth and the Moon, both of which have different gravitational accelerations. Out in deep space, away from all gravitational effects, a mass of 1 tonne will weigh nothing! |
|
Weld Joint Factor |
A utilisation factor for the strength of a welded joint A weld joint factor of 1.0 is usual for coded welders using certified procedures and materials The lowest WJF for a weld completed by an experienced, but uncoded welder using uncertified procedures and materials is ≈0.6 |
|
Wire Rope |
A rope comprising at least one strand, which comprises more than one filament made from metal Also recognised generically as: wire, steel rope, multi-strand wire, flexible wire, cable, cord and steelcord |
|
Work |
A quantity synonymous with energy Mechanical: a force applied over a distance greater than zero As stated above, work is defined as force x distance {N.m} The greater the force or energy or the further the distance the more work has been done The units of measurement are; Btu, W.h, J, cal, N.m, lbf.ft, C, etc. |
|
Work Harden |
The process of through-hardening a steel or a steel alloy by repeated deformation at stresses greater than yield stress However, if the steel/alloy is subsequently held at a sufficiently high temperature for a long enough period it will eventually revert to its original properties |
|
Wrap |
The longitudinal helical coiling of filaments into strands or strands into wire rope during the manufacturing process |
|
X-Rays |
Short-wave electromagnetic radiation that falls between ultra-violet light and gamma radiation Ranges from: |
|
Yaw (vessels) |

The rotational movement of a vessel about the vertical ('z') axis Whilst this term generally applies to the movement of ships, it is also used to define the movement of any object subject to the same degrees of freedom; e.g. aircraft and spacecraft see Right-Hand Rule for positive and negative directions of this movement |
|
Yield Stress |
The maximum stress that can be applied to a metal whilst that continues to obey Hooke's Law The release of stress above yield stress will result in permanent deformation of the material see also Specified Minimum Yield Stress |
|
Young's Modulus |
The linear relationship (the slope of the curve) between purely elastic stress and strain in a material |
|
Z Boson |
A weak force carrier There is one type of Z Boson, which only changes spin and momentum but cannot change either electric charge nor any other charges (like strangeness, charm, etc.), so it never changes the generation or flavour of the particle emitting it. The Z boson (or 'Z') is electrically neutral and is its own anti-particle m=1.62556647E−22g [91.1876GeV/c²], lifetime≈1E-25s, Q=0, Iz=1 |
'ε': Owing to the scientific work completed by Keith Dixon-Roche over recent years, CalQlata has concluded that a number of the conventional definitions [originally] provided above are little more than unsupported guesswork based upon flawed assumptions (e.g. light possesses mass). Whilst they remain in this webpage, they are now provided for information only.